Difference between revisions of "Polymethyl methacrylate"
(username removed) |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Hard, glasslike, thermoplastic resin with a softening temperature from 105-125 C. Polymethyl methacrylate was the first commercially sold in 1933 as glass substitutes, such as [ | + | Hard, glasslike, thermoplastic resin with a softening temperature from 105-125 C. Polymethyl methacrylate was the first commercially sold in 1933 as glass substitutes, such as [[Plexiglas|Plexiglas®]], [[Perspex|Perspex]], and [[Lucite|Lucite®]]. Polymethyl methacrylate has good optical properties and is often used as a replacement for glass. It is resistant to oxidation and photodegradation. |
| − | |||
| − | |||
| + | See also [[acrylic%20resin|acrylic resin]]. | ||
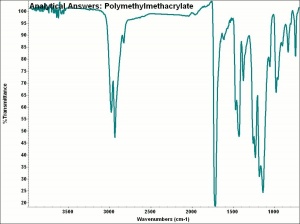
| + | [[[SliderGallery rightalign|aaiPMMA.jpg~FTIR]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 11: | Line 11: | ||
Examples: Lucite® [Lucite]; Perspex® [Lucite); Plexiglas® [Arkema]; Altuglas® [Arkema]; Acrylite® [Piedmont Plastics] | Examples: Lucite® [Lucite]; Perspex® [Lucite); Plexiglas® [Arkema]; Altuglas® [Arkema]; Acrylite® [Piedmont Plastics] | ||
| − | + | == Physical and Chemical Properties == | |
| − | |||
| − | == | ||
| − | Burns with a shiny flame and blue center; smells sweet and fruity. Soluble in esters, ketones, aromatic and chlorinated hydrocarbons. Insoluble in water, alcohols, petroleum hydrocarbons. Brinell hardness=18-20 | + | * Burns with a shiny flame and blue center; smells sweet and fruity. |
| + | * Soluble in esters, ketones, aromatic and chlorinated hydrocarbons. Insoluble in water, alcohols, petroleum hydrocarbons. | ||
| + | * Brinell hardness=18-20 | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 26: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 105-125 (softens) | + | | 105-125 C(softens) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.16-1.20 | + | | 1.16-1.20 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 41: | Line 41: | ||
[[media:download_file_355.pdf|Physical Properties for Selected Thermoplastic Resins]] | [[media:download_file_355.pdf|Physical Properties for Selected Thermoplastic Resins]] | ||
| − | + | == Resources and Citations == | |
| − | + | * Omnexus: [https://omnexus.specialchem.com/selection-guide/polymethyl-methacrylate-pmma-acrylic-plastic Guide on PMMA] | |
| − | == | ||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | ||
| − | |||
* Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | ||
| − | |||
* Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | ||
| − | + | * Wikipedia: http://en.wikipedia.org/wiki/Plexiglas (Accessed Nov. 9, 2005) | |
| − | * Wikipedia | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:38, 18 September 2023
Description
Hard, glasslike, thermoplastic resin with a softening temperature from 105-125 C. Polymethyl methacrylate was the first commercially sold in 1933 as glass substitutes, such as Plexiglas®, Perspex, and Lucite®. Polymethyl methacrylate has good optical properties and is often used as a replacement for glass. It is resistant to oxidation and photodegradation.
See also Acrylic resin.
Synonyms and Related Terms
PMMA; acrylic resin; polymethylmethacrylate; polimetilmetacrilato (Esp.); poli(metacrilato de metilo) (Esp.); polyméthylméthacrylate (Fr.); polimetilmetacrilato (It.); polimetilmetacrilato (Port.)
Examples: Lucite® [Lucite]; Perspex® [Lucite); Plexiglas® [Arkema]; Altuglas® [Arkema]; Acrylite® [Piedmont Plastics]
Physical and Chemical Properties
- Burns with a shiny flame and blue center; smells sweet and fruity.
- Soluble in esters, ketones, aromatic and chlorinated hydrocarbons. Insoluble in water, alcohols, petroleum hydrocarbons.
- Brinell hardness=18-20
| Composition | (C5O2H8)n |
|---|---|
| CAS | 9011-14-7 |
| Melting Point | 105-125 C(softens) |
| Density | 1.16-1.20 g/ml |
| Refractive Index | 1.482-1.521 |
Comparisons
General Characteristics of Polymers
Physical Properties for Selected Thermoplastic Resins
Resources and Citations
- Omnexus: Guide on PMMA
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Wikipedia: http://en.wikipedia.org/wiki/Plexiglas (Accessed Nov. 9, 2005)