Difference between revisions of "Fluorescein"
Jump to navigation
Jump to search
(username removed) |
|||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A bright, orange to red crystalline powder that reacts with alkalis to form an intense green [ | + | A bright, orange to red crystalline powder that reacts with alkalis to form an intense green [[fluorescence]]. Fluorescein is used for the detection of soluble hydroxides, carbonates, and ammonia. It is sensitive to the part per billion level. Its UV absorption maxima occur at 493.4 and 460 nm. Fluorescein is used as an indicator and as a textile dye. In the late 19th century, fluorescein was used as a yellow dye on [[wool]] and [[silk]] without a mordant. |
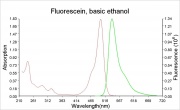
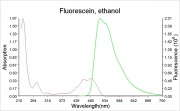
[[File:Fluorescein_ethanol_abs.ems.jpg|thumb|Absorption and fluorescence emission spectra]] | [[File:Fluorescein_ethanol_abs.ems.jpg|thumb|Absorption and fluorescence emission spectra]] | ||
| + | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Solvent Yellow 94; CI 45350:1; D&C Yellow No.7; 3',6'-dihydroxyspiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one; resorcinolphthalein; Fluorescein (Deut.); fluorescéïne (Fr.); diresorcinolphthalein; | Solvent Yellow 94; CI 45350:1; D&C Yellow No.7; 3',6'-dihydroxyspiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one; resorcinolphthalein; Fluorescein (Deut.); fluorescéïne (Fr.); diresorcinolphthalein; | ||
| − | == | + | == Risks == |
| − | + | * Combustible. | |
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC119241000&productDescription=FLUORESCEIN+100GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| − | The fluorescence of fluorescein changes with pH (colorless below pH=4.0 and fluorescent green above 4.5) | + | ==Physical and Chemical Properties== |
| + | |||
| + | * Soluble in hot ethanol, glacial acetic acid. Soluble in alkalis and ammonia producing a bright green fluorescence. Insoluble in water, benzene, chloroform, ether. | ||
| + | * The fluorescence of fluorescein changes with pH (colorless below pH=4.0 and fluorescent green above 4.5) | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 314-316 | + | | 314-316 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 30: | Line 35: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 10:11, 25 July 2022
Description
A bright, orange to red crystalline powder that reacts with alkalis to form an intense green Fluorescence. Fluorescein is used for the detection of soluble hydroxides, carbonates, and ammonia. It is sensitive to the part per billion level. Its UV absorption maxima occur at 493.4 and 460 nm. Fluorescein is used as an indicator and as a textile dye. In the late 19th century, fluorescein was used as a yellow dye on Wool and Silk without a mordant.
Synonyms and Related Terms
Solvent Yellow 94; CI 45350:1; D&C Yellow No.7; 3',6'-dihydroxyspiro[isobenzofuran-1(3H),9'-[9H]xanthen]-3-one; resorcinolphthalein; Fluorescein (Deut.); fluorescéïne (Fr.); diresorcinolphthalein;
Risks
- Combustible.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in hot ethanol, glacial acetic acid. Soluble in alkalis and ammonia producing a bright green fluorescence. Insoluble in water, benzene, chloroform, ether.
- The fluorescence of fluorescein changes with pH (colorless below pH=4.0 and fluorescent green above 4.5)
| Composition | C20H12O5 |
|---|---|
| CAS | 2321-07-2 |
| Melting Point | 314-316 C |
| Molecular Weight | mol. wt. = 332.32 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 4194
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: The fluorescence of fluorescein changes with pH. It is colorless below pH=4.0 and above 4.5, it fluoresces green.
- Sigma Dyes, Stains and Natural Pigments, Infrared Library, Nicolet, 1991-1995
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876 Comment: p. 251