Difference between revisions of "Atacamite"
| (5 intermediate revisions by 3 users not shown) | |||
| Line 7: | Line 7: | ||
copper oxychloride; Atacamit (Deut.); atacamite (Fr., It., Port.); atacamita (Esp.); atakamitis (Gr.); atacamiet (Ned.); wild patina; Mehlpatina; flour patina; peste du bronze; bronze disease; malignant patina; rogna; caries; | copper oxychloride; Atacamit (Deut.); atacamite (Fr., It., Port.); atacamita (Esp.); atakamitis (Gr.); atacamiet (Ned.); wild patina; Mehlpatina; flour patina; peste du bronze; bronze disease; malignant patina; rogna; caries; | ||
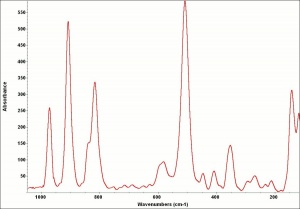
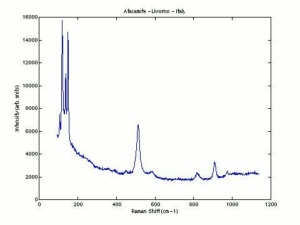
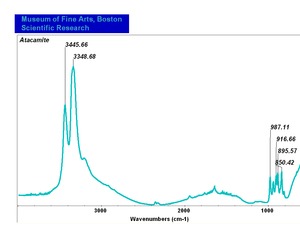
| − | [[[SliderGallery rightalign|AtacamitUCL.jpg~Raman|Atacamiteitaly1.jpg~Raman| | + | [[[SliderGallery rightalign|AtacamitUCL.jpg~Raman (UCL)|Atacamiteitaly1.jpg~Raman (U of Parma)|Atacamite.TIF~FTIR (MFA)]]] |
| − | == | + | == Physical and Chemical Properties == |
Crystallizes as thin orthorhombic prisms; Brittle with conchoidal fracture; Luster = adamantine to vitreous; Streak = green; Transparent to translucent. | Crystallizes as thin orthorhombic prisms; Brittle with conchoidal fracture; Luster = adamantine to vitreous; Streak = green; Transparent to translucent. | ||
| Line 22: | Line 22: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.76-3.78 | + | | 3.76-3.78 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 31: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | M.Naumova, S.Pisareva,"A Note on the Use of Blue and Green Copper Compounds in Paintings" ''Studies in Conservation'' 39:277-283, 1994. | + | * M.Naumova, S.Pisareva,"A Note on the Use of Blue and Green Copper Compounds in Paintings" ''Studies in Conservation'' 39:277-283, 1994. |
| − | |||
| − | |||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| Line 43: | Line 41: | ||
* Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 | ||
| − | * | + | * H. Otto, "X-ray fine structure investigation of patina samples" Freiberger Forschungshefte.B, 37, 1959, pp. 66-77. |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 233 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 233 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Atacamite (accessed Aug 30 2005) |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Latest revision as of 12:33, 9 December 2022
Description
A green mineral composed of Copper oxychloride. Atacamite was named by D. de Fallizen in 1801 when he identified it as a mineral found in the Chilean Atacama Desert. It occurs naturally in the oxidation zones of copper deposits and has been found in Chile, China, Russia, Czech Republic, Arizona, and Australia. It ranges in color from a soft muted green to emerald to blackish green. Atacamite also occurs as a chloride corrosion product on Copper and Bronze objects. Its presence indicates bronze disease. Using this corrosion reaction, Theophilus gave a recipe for the synthetic preparation of atacamite (Naumova and Pisareva, 1992). Atacamite has been found as a green pigment on sculpture, manuscripts, maps, and frescoes painted in Asia, Russia, Persia and Europe.
Synonyms and Related Terms
copper oxychloride; Atacamit (Deut.); atacamite (Fr., It., Port.); atacamita (Esp.); atakamitis (Gr.); atacamiet (Ned.); wild patina; Mehlpatina; flour patina; peste du bronze; bronze disease; malignant patina; rogna; caries;
Physical and Chemical Properties
Crystallizes as thin orthorhombic prisms; Brittle with conchoidal fracture; Luster = adamantine to vitreous; Streak = green; Transparent to translucent.
| Composition | Cu2Cl(OH)3 |
|---|---|
| Mohs Hardness | 3.0 - 3.5 |
| Density | 3.76-3.78 g/ml |
| Molecular Weight | 213.57 |
| Refractive Index | 1.831; 1.861; 1.880 |
Resources and Citations
- M.Naumova, S.Pisareva,"A Note on the Use of Blue and Green Copper Compounds in Paintings" Studies in Conservation 39:277-283, 1994.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- H. Otto, "X-ray fine structure investigation of patina samples" Freiberger Forschungshefte.B, 37, 1959, pp. 66-77.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 233
- Wikipedia: http://en.wikipedia.org/wiki/Atacamite (accessed Aug 30 2005)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997