Difference between revisions of "Benzotriazole"
| (One intermediate revision by one other user not shown) | |||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|MFA- Benzotriazole.jpg~FTIR|benzotiazole.jpg~Chemical structure]]] | [[[SliderGallery rightalign|MFA- Benzotriazole.jpg~FTIR|benzotiazole.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Moderately toxic by ingestion and inhalation. | ||
| + | * Skin contact may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AAA1542318&productDescription=BENZOTRIAZOLE%2C+99%25+%28ASSAY%29+50G&vendorId=VN00024248&countryCode=US&language=en SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Soluble in ethanol, benzene, chloroform, dimethylformamide, toluene. Slightly soluble in water. Stable in acids and alkalis. | Soluble in ethanol, benzene, chloroform, dimethylformamide, toluene. Slightly soluble in water. Stable in acids and alkalis. | ||
| Line 24: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 98-100 | + | | 98-100 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.36 | + | | 1.36 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 39: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 201-204 | + | | 201-204 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * H.B.Madsen "A Preliminary Note on the Use of BTA for Stabilizing Bronze Objects" ''Studies in Conservation'' 12: 163-167, 1967. | |
| − | + | * C.Sease, "Benzotriazole: A Review for Conservators" ''Studies in Conservation'' 23:76-85, 1978. | |
| − | + | * H.B.Madsen "Further Remarks on the Use of Benzotriazole for Stabilizing Bronze Objects" ''Studies in Conservation'' 16:120-122, 1971 | |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 60: | Line 58: | ||
* Marie Svoboda, Conservation Survey Index, unpublished, 1997 | * Marie Svoboda, Conservation Survey Index, unpublished, 1997 | ||
| − | * | + | * Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:30, 4 May 2022
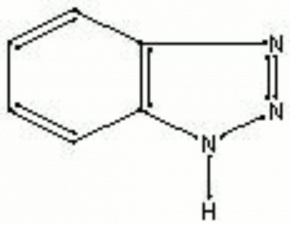
Description
A light tan, crystalline powder that reacts with metals to form stable salts. Benzotriazole is used in industry as a fixing agent in photographic emulsions, as an antitarnish agent for copper and its alloys, and as a corrosion inhibitor in antifreezes and water coolant systems. In 1967, it was used to prevent corrosion on bronze works of art as an additive in an acrylic resin formulation called Incralac (Madsen 1967). More recently benzotriazole has also been used as a Vapor phase corrosion inhibitor and as an additive in antitarnish cloths. Benzotriazole is also used as an antifoggant in photographic developers.
Synonyms and Related Terms
1,2,3-benzotriazole; 1H-benzotriazole; BTA; aziminobenzene; benzene azimide; benzisotriazole; Cobratec 99; 1,2-aminozophenylene;
Risks
- Moderately toxic by ingestion and inhalation.
- Skin contact may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in ethanol, benzene, chloroform, dimethylformamide, toluene. Slightly soluble in water. Stable in acids and alkalis.
Vapor pressure = 0.04 @ 20C
| Composition | C6H6NHN2 |
|---|---|
| CAS | 95-14-7 |
| Melting Point | 98-100 C |
| Density | 1.36 g/ml |
| Molecular Weight | mol. wt.=119.12 |
| Boiling Point | 201-204 C |
Resources and Citations
- H.B.Madsen "A Preliminary Note on the Use of BTA for Stabilizing Bronze Objects" Studies in Conservation 12: 163-167, 1967.
- C.Sease, "Benzotriazole: A Review for Conservators" Studies in Conservation 23:76-85, 1978.
- H.B.Madsen "Further Remarks on the Use of Benzotriazole for Stabilizing Bronze Objects" Studies in Conservation 16:120-122, 1971
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: Entry # 1140
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Marie Svoboda, Conservation Survey Index, unpublished, 1997
- Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm