Difference between revisions of "Boric acid"
Jump to navigation
Jump to search
| (One intermediate revision by one other user not shown) | |||
| Line 2: | Line 2: | ||
A white crystalline powder that occurs in nature as the mineral sassolite. Commercially, boric acid is primarily synthesized from [[borax]]. It is used to weatherproof wood and fireproof fabrics. It is also used to produce glass, enamels, and porcelain. Boric acid is used as an [[insecticide]] for [[cockroach|cockroaches]], [[silverfish]], and [[black%20carpet%20beetle|black carpet beetles]] usually in a mixture with silica dust called B + S. It has been used as an antibacterial eyewash, a buffer in photo processing solutions, and a mordant for printing and dyeing. | A white crystalline powder that occurs in nature as the mineral sassolite. Commercially, boric acid is primarily synthesized from [[borax]]. It is used to weatherproof wood and fireproof fabrics. It is also used to produce glass, enamels, and porcelain. Boric acid is used as an [[insecticide]] for [[cockroach|cockroaches]], [[silverfish]], and [[black%20carpet%20beetle|black carpet beetles]] usually in a mixture with silica dust called B + S. It has been used as an antibacterial eyewash, a buffer in photo processing solutions, and a mordant for printing and dyeing. | ||
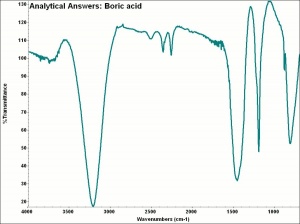
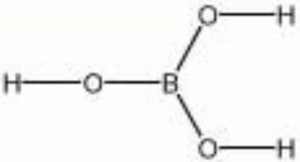
| − | + | [[[SliderGallery rightalign|aaiACID_BOR.jpg~FTIR|boric acid.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
boracic acid; orthoboric acid; acide borique (Fr.); borsaure; NC1-C56417; Borofax; B + S ; Kill-Off; Boozuur | boracic acid; orthoboric acid; acide borique (Fr.); borsaure; NC1-C56417; Borofax; B + S ; Kill-Off; Boozuur | ||
| − | [ | + | == Risks == |
| + | |||
| + | * May cause toxicity by ingestion and skin contact. LD50 = 5000 mg/kg | ||
| + | * Noncombustible. | ||
| + | * ThermoFisher: [https://www.fishersci.com/msds?productName=AC315181000 SDS] | ||
| − | == | + | ==Physical and Chemical Properties== |
Soluble in water, ethanol, glycerol. | Soluble in water, ethanol, glycerol. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 171 (dec) | + | | 171 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.4347 | + | | 1.4347 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 35: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Nancy Odegaard, Alyce Sadongei, and associates, ''Old Poisons, New Problems'', Altimira, Walnut Creek, CA, 2005 | * Nancy Odegaard, Alyce Sadongei, and associates, ''Old Poisons, New Problems'', Altimira, Walnut Creek, CA, 2005 | ||
| Line 63: | Line 59: | ||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| − | * | + | * Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 07:26, 10 May 2022
Description
A white crystalline powder that occurs in nature as the mineral sassolite. Commercially, boric acid is primarily synthesized from Borax. It is used to weatherproof wood and fireproof fabrics. It is also used to produce glass, enamels, and porcelain. Boric acid is used as an Insecticide for cockroaches, Silverfish, and black carpet beetles usually in a mixture with silica dust called B + S. It has been used as an antibacterial eyewash, a buffer in photo processing solutions, and a mordant for printing and dyeing.
Synonyms and Related Terms
boracic acid; orthoboric acid; acide borique (Fr.); borsaure; NC1-C56417; Borofax; B + S ; Kill-Off; Boozuur
Risks
- May cause toxicity by ingestion and skin contact. LD50 = 5000 mg/kg
- Noncombustible.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, ethanol, glycerol.
| Composition | H3BO3 |
|---|---|
| CAS | 10043-35-3 |
| Melting Point | 171 C (dec) |
| Density | 1.4347 g/ml |
| Molecular Weight | mol. wt. = 61.8 |
Resources and Citations
- Nancy Odegaard, Alyce Sadongei, and associates, Old Poisons, New Problems, Altimira, Walnut Creek, CA, 2005
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1364
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 109
- Lynda A. Zycherman, J.Richard Schrock, A Guide to Museum Pest Control, FAIC and Association of Systematics Collections, Washington DC, 1988
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- G.Caneva, M.P.Nugari, O.Salvadori, Biology in the Conservation of Works of Art, ICCROM, Rome, 1991
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Photographic chemicals: www.jetcity.com/~mrjones/chemdesc.htm