Difference between revisions of "Chalcanthite"
Jump to navigation
Jump to search
| (6 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:image1_chalcanthite.jpg|thumb|Chalcanthite]] | [[File:image1_chalcanthite.jpg|thumb|Chalcanthite]] | ||
== Description == | == Description == | ||
| − | + | [[File:pc31220chalcanthite.jpg|thumb|Chalcanthite]] | |
A rare, azure blue mineral composed of hydrous [[copper sulfate]]. Chalcanthite is found as a secondary mineral in the oxidized zones of copper sulfide ore deposits in Chile and the U.S.(Nevada, Tennessee, Utah). It can be transparent to translucent. | A rare, azure blue mineral composed of hydrous [[copper sulfate]]. Chalcanthite is found as a secondary mineral in the oxidized zones of copper sulfide ore deposits in Chile and the U.S.(Nevada, Tennessee, Utah). It can be transparent to translucent. | ||
| Line 8: | Line 8: | ||
copper sulfate pentahydrate; blue vitriol; blue stone; bluestone; copper vitriol; calcantita (Esp.) | copper sulfate pentahydrate; blue vitriol; blue stone; bluestone; copper vitriol; calcantita (Esp.) | ||
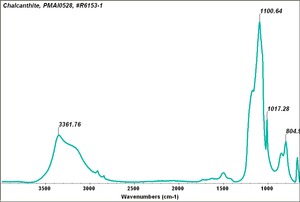
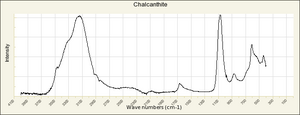
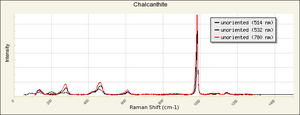
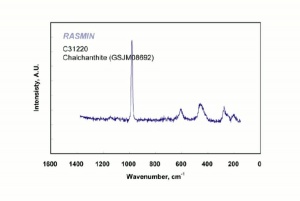
| − | [[[SliderGallery rightalign|chalcanthiteRS.jpg~Raman]]] | + | [[[SliderGallery rightalign|Chalcanthite, PMAI0528, -R6153-1.TIF~FTIR (PMA)|Chalcanthite Infrared R050354 RRUFF.png~IR-ATR (RRUFF)|Chalcanthite Raman R050354 RRUFF.png~Raman (RRUFF)|chalcanthiteRS.jpg~Raman (RASMIN)]]] |
| + | == Risks == | ||
| − | + | * Ingestion is toxic. | |
| − | + | == Physical and Chemical Properties == | |
| − | Soluble in water. | + | * Triclinic, short prismatic crystals. |
| + | * Fracture = conchoidal. | ||
| + | * Luster = vitreous. | ||
| + | * Streak = white | ||
| + | * Soluble in water. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 25: | Line 30: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.286 | + | | 2.286 g/ml |
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | + | * Mineralogy Database: [http://www.webmineral.com/data/Chalcanthite.shtml Chalcanthite | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | Mineralogy Database: [http://www.webmineral.com/data/Chalcanthite.shtml Chalcanthite | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 235 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 235 | ||
| Line 51: | Line 42: | ||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Chalcanthite (Accessed Sept 2 2005; spec. grav=2.12-2.3 ) |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Latest revision as of 13:26, 6 December 2022
Description
A rare, azure blue mineral composed of hydrous Copper sulfate. Chalcanthite is found as a secondary mineral in the oxidized zones of copper sulfide ore deposits in Chile and the U.S.(Nevada, Tennessee, Utah). It can be transparent to translucent.
Synonyms and Related Terms
copper sulfate pentahydrate; blue vitriol; blue stone; bluestone; copper vitriol; calcantita (Esp.)
Risks
- Ingestion is toxic.
Physical and Chemical Properties
- Triclinic, short prismatic crystals.
- Fracture = conchoidal.
- Luster = vitreous.
- Streak = white
- Soluble in water.
| Composition | CuSO4 - 5H2O |
|---|---|
| Mohs Hardness | 2.5 |
| Density | 2.286 g/ml |
Resources and Citations
- Mineralogy Database: [http://www.webmineral.com/data/Chalcanthite.shtml Chalcanthite
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 235
- Henry Hodges, Artifacts: An Introduction to Early Materials and Technology, Ronald P. Frye, Kingston, Canada, 1988
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: http://en.wikipedia.org/wiki/Chalcanthite (Accessed Sept 2 2005; spec. grav=2.12-2.3 )
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997