Difference between revisions of "Cobalt green"
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:420 cobalt green.jpg|thumb|Cobalt green]] | [[File:420 cobalt green.jpg|thumb|Cobalt green]] | ||
== Description == | == Description == | ||
| − | + | [[File:cogreen C100x.jpg|thumb|Cobalt green]] | |
A composite green pigment composed of cobalt and zinc oxides that have been calcined together. First developed in 1780 by Sven Rinman, a Swedish chemist, cobalt green, or Rinman's green, was not sold as an artists pigment in 1835. Cobalt green is a permanent, bright, bluish-green pigment. It is a good drier in oil paints but has low tinctorial power. The name cobalt green has also been used for some commercial paints that have a mixture of [[cobalt blue]] and [[chrome yellow]]. | A composite green pigment composed of cobalt and zinc oxides that have been calcined together. First developed in 1780 by Sven Rinman, a Swedish chemist, cobalt green, or Rinman's green, was not sold as an artists pigment in 1835. Cobalt green is a permanent, bright, bluish-green pigment. It is a good drier in oil paints but has low tinctorial power. The name cobalt green has also been used for some commercial paints that have a mixture of [[cobalt blue]] and [[chrome yellow]]. | ||
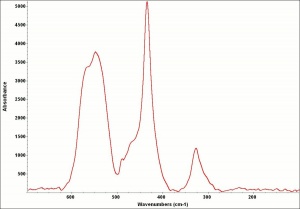
| − | [[ | + | [[[SliderGallery rightalign|CobaltgrUCL.jpg~Raman]]] |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 10: | Line 10: | ||
Pigment Green 19; CI 77335; verde cobalto (Esp.); Kobaltgrün (Deut.); Rinmansgrün (Deut.); vert de cobalt (Fr.); prasino toy kobaltioy (Gr.); verde di cobalto (It.); verde di zinco (It.); cobaltgroen (Ned.); verde de cobalto (Port.); zinc green (incorrect); Rinman's green; Rinmann's green; Saxony green; Swedish green; smalt green; Gellert green; | Pigment Green 19; CI 77335; verde cobalto (Esp.); Kobaltgrün (Deut.); Rinmansgrün (Deut.); vert de cobalt (Fr.); prasino toy kobaltioy (Gr.); verde di cobalto (It.); verde di zinco (It.); cobaltgroen (Ned.); verde de cobalto (Port.); zinc green (incorrect); Rinman's green; Rinmann's green; Saxony green; Swedish green; smalt green; Gellert green; | ||
| − | + | == Risks == | |
| − | |||
| − | == | ||
| − | + | * Skin contact may cause allergies, especially on elbows, neck and ankles. | |
| + | * Chronic inhalation may cause asthma. | ||
| + | * Ingestion may cause vomiting, diarrhea and the sensation of hotness. | ||
| + | * Kremer-Pigmente: [https://www.kremer-pigmente.com/en/shop/pigments/44101-cobalt-green-pg-50.html SDS] | ||
| − | + | ==Physical and Chemical Properties== | |
| − | Resistant to alkalis. Slightly soluble in acids producing a pale pink solution. | + | * Fine, regular, rounded, transparent particles. |
| + | * Bright green in transmitted light. | ||
| + | * Highly refraction. | ||
| + | * High birefringence. | ||
| + | * Resistant to alkalis. | ||
| + | * Slightly soluble in acids producing a pale pink solution. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 29: | Line 35: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Pigments Through the Ages: [http://webexhibits.org/pigments/indiv/overview/cogreen.html Azurite] | |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: p. 109 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: p. 109 | ||
| Line 62: | Line 62: | ||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | |||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:00, 30 May 2022
Description
A composite green pigment composed of cobalt and zinc oxides that have been calcined together. First developed in 1780 by Sven Rinman, a Swedish chemist, cobalt green, or Rinman's green, was not sold as an artists pigment in 1835. Cobalt green is a permanent, bright, bluish-green pigment. It is a good drier in oil paints but has low tinctorial power. The name cobalt green has also been used for some commercial paints that have a mixture of Cobalt blue and Chrome yellow.
Synonyms and Related Terms
Pigment Green 19; CI 77335; verde cobalto (Esp.); Kobaltgrün (Deut.); Rinmansgrün (Deut.); vert de cobalt (Fr.); prasino toy kobaltioy (Gr.); verde di cobalto (It.); verde di zinco (It.); cobaltgroen (Ned.); verde de cobalto (Port.); zinc green (incorrect); Rinman's green; Rinmann's green; Saxony green; Swedish green; smalt green; Gellert green;
Risks
- Skin contact may cause allergies, especially on elbows, neck and ankles.
- Chronic inhalation may cause asthma.
- Ingestion may cause vomiting, diarrhea and the sensation of hotness.
- Kremer-Pigmente: SDS
Physical and Chemical Properties
- Fine, regular, rounded, transparent particles.
- Bright green in transmitted light.
- Highly refraction.
- High birefringence.
- Resistant to alkalis.
- Slightly soluble in acids producing a pale pink solution.
| Composition | CoO-ZnO |
|---|---|
| Refractive Index | 1.94-2.0 |
Resources and Citations
- Pigments Through the Ages: Azurite
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: p. 109
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- David Bomford, Jo Kirby, John Leighton, Ashok Roy, Art in the Making:Impressionism, National Gallery, London, 1990
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000