Difference between revisions of "Ethyl acrylate"
| (One intermediate revision by one other user not shown) | |||
| Line 2: | Line 2: | ||
A colorless, acrid smelling liquid primarily used as a monomer for the production as [[acrylic resin|acrylic]] polymers. Ethyl acrylate self-polymerizes at room temperature and occurs more rapidly with the aid of elevated temperatures, light, or peroxides. Polyethyl acrylate is a transparent, slightly elastic resin that is resistant to most [[solvent|solvents]]. Ethyl acrylate monomer is used in water [[emulsion]] paints and for coatings on [[textile|textiles]], [[paper]], and [[leather]]. | A colorless, acrid smelling liquid primarily used as a monomer for the production as [[acrylic resin|acrylic]] polymers. Ethyl acrylate self-polymerizes at room temperature and occurs more rapidly with the aid of elevated temperatures, light, or peroxides. Polyethyl acrylate is a transparent, slightly elastic resin that is resistant to most [[solvent|solvents]]. Ethyl acrylate monomer is used in water [[emulsion]] paints and for coatings on [[textile|textiles]], [[paper]], and [[leather]]. | ||
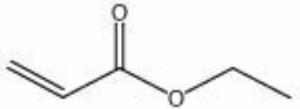
| − | + | [[[SliderGallery rightalign|ethyl acrylate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
2-propenoic acid ethyl ester; acrylic acid ethyl ester; acrilato de etilo (Esp.); 2-propenoato de etilo (Esp.); acrylate d'éthyle (Fr.); etil acrilato (It.); acrilato de etilo (Port.) | 2-propenoic acid ethyl ester; acrylic acid ethyl ester; acrilato de etilo (Esp.); 2-propenoato de etilo (Esp.); acrylate d'éthyle (Fr.); etil acrilato (It.); acrilato de etilo (Port.) | ||
| − | |||
| − | == | + | |
| + | ==Physical and Chemical Properties== | ||
Soluble in ethanol, ether. | Soluble in ethanol, ether. | ||
| Line 22: | Line 22: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -72 | + | | -72 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.9405 | + | | 0.9405 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 31: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 99.4 | + | | 99.4 C |
|} | |} | ||
| − | == | + | == Risks == |
Toxic by ingestion, inhalation and skin contact. Skin contact will cause irritation. Suspected carcinogen. Flammable. Flash point = 9C | Toxic by ingestion, inhalation and skin contact. Skin contact will cause irritation. Suspected carcinogen. Flammable. Flash point = 9C | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3805 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3805 | ||
Latest revision as of 13:59, 5 August 2022
Description
A colorless, acrid smelling liquid primarily used as a monomer for the production as acrylic polymers. Ethyl acrylate self-polymerizes at room temperature and occurs more rapidly with the aid of elevated temperatures, light, or peroxides. Polyethyl acrylate is a transparent, slightly elastic resin that is resistant to most solvents. Ethyl acrylate monomer is used in water Emulsion paints and for coatings on textiles, Paper, and Leather.
Synonyms and Related Terms
2-propenoic acid ethyl ester; acrylic acid ethyl ester; acrilato de etilo (Esp.); 2-propenoato de etilo (Esp.); acrylate d'éthyle (Fr.); etil acrilato (It.); acrilato de etilo (Port.)
Physical and Chemical Properties
Soluble in ethanol, ether.
| Composition | CH2:CHCOOC2H5 |
|---|---|
| CAS | 140-88-5 |
| Melting Point | -72 C |
| Density | 0.9405 g/ml |
| Molecular Weight | mol. wt. = 100.1 |
| Boiling Point | 99.4 C |
Risks
Toxic by ingestion, inhalation and skin contact. Skin contact will cause irritation. Suspected carcinogen. Flammable. Flash point = 9C
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3805
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993