Difference between revisions of "Eosin"
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:MFA2018450 Eosin.jpg|thumb|Chinese drawing<br>MFA# 2018.450]] | ||
| + | == Description == | ||
[[File:Eosin.jpg|thumb|Eosin]] | [[File:Eosin.jpg|thumb|Eosin]] | ||
| − | |||
| − | |||
A red crystalline dye composed of the [[potassium]], [[sodium]], or [[lead]] salt of tetrabromofluorescein. First discovered by Caro in 1871, eosin is primarily used as an acid dye for producing a blood red color in [[silk]], [[wool]], [[paper]], [[leather]], and [[cotton]]. It is also used as a histological stain, a cosmetic colorant, and a colorant in red inks. In the late 19th and early 20th century, eosin was also used as a red paint pigment. Alcoholic solutions have a strong green [[autofluorescence]]. Eosin is not permanent and fades rapidly in sunlight. | A red crystalline dye composed of the [[potassium]], [[sodium]], or [[lead]] salt of tetrabromofluorescein. First discovered by Caro in 1871, eosin is primarily used as an acid dye for producing a blood red color in [[silk]], [[wool]], [[paper]], [[leather]], and [[cotton]]. It is also used as a histological stain, a cosmetic colorant, and a colorant in red inks. In the late 19th and early 20th century, eosin was also used as a red paint pigment. Alcoholic solutions have a strong green [[autofluorescence]]. Eosin is not permanent and fades rapidly in sunlight. | ||
| Line 9: | Line 9: | ||
tetrabromofluorescein; Pigment Red 90; CI 45380; D&C Red No. 22; D&C Red No. 21; Acid Red 87; Solvent Red 43; éosine (Fr.); eosina (Esp., Port.); eosin Y; eosine A; eosine G; bromeosin; bromofluoresceic acid; geranium lake; eosine yellowish | tetrabromofluorescein; Pigment Red 90; CI 45380; D&C Red No. 22; D&C Red No. 21; Acid Red 87; Solvent Red 43; éosine (Fr.); eosina (Esp., Port.); eosin Y; eosine A; eosine G; bromeosin; bromofluoresceic acid; geranium lake; eosine yellowish | ||
| + | == Risks == | ||
| − | + | * Causes skin irritation. | |
| − | + | * Fades rapidly in sunlight. | |
| − | + | * Delasco: [https://www.delasco.com/content/sds/EOSIN-SDS.pdf SDS] | |
| − | + | == Physical and Chemical Properties == | |
| − | Maximum emission wavelength = 540 nm. | + | * Soluble in ethanol. Insoluble in water (potassium and sodium salts of eosin are water soluble). |
| + | * Maximum absorption wavelength = 520 nm. | ||
| + | * Maximum emission wavelength = 540 nm. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 27: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 300 | + | | 300 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 36: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * M.Ballard (ed.), ''Important Early Synthetic Dyes. Chemistry, Constitution, Date, Properties.'' Conservation Analytical Laboratory, Smithsonian Institution, 1991. | |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 53: | Line 48: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3639 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3639 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Chemical Compound." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Chemical Compound."(Accessed 12 May 2004). |
| − | * Website | + | * Website: www.probes.com/handbook/sections - gives absorption max=524 and emission max=544 |
| − | * Website | + | * Website: member.pgonline.com/~bryand/dyes/45380.htm - absorption max = 516 |
* Colour Index International online at www.colour-index.org Comment: discoverer and date | * Colour Index International online at www.colour-index.org Comment: discoverer and date | ||
Latest revision as of 07:30, 2 August 2022
Description
A red crystalline dye composed of the Potassium, Sodium, or Lead salt of tetrabromofluorescein. First discovered by Caro in 1871, eosin is primarily used as an acid dye for producing a blood red color in Silk, Wool, Paper, Leather, and Cotton. It is also used as a histological stain, a cosmetic colorant, and a colorant in red inks. In the late 19th and early 20th century, eosin was also used as a red paint pigment. Alcoholic solutions have a strong green Autofluorescence. Eosin is not permanent and fades rapidly in sunlight.
Synonyms and Related Terms
tetrabromofluorescein; Pigment Red 90; CI 45380; D&C Red No. 22; D&C Red No. 21; Acid Red 87; Solvent Red 43; éosine (Fr.); eosina (Esp., Port.); eosin Y; eosine A; eosine G; bromeosin; bromofluoresceic acid; geranium lake; eosine yellowish
Risks
- Causes skin irritation.
- Fades rapidly in sunlight.
- Delasco: SDS
Physical and Chemical Properties
- Soluble in ethanol. Insoluble in water (potassium and sodium salts of eosin are water soluble).
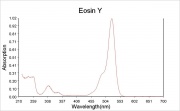
- Maximum absorption wavelength = 520 nm.
- Maximum emission wavelength = 540 nm.
| Composition | C20H8O5Br4 |
|---|---|
| CAS | 548-26-5 |
| Melting Point | 300 C |
| Molecular Weight | Mol. wt. = 647.9 |
Resources and Citations
- M.Ballard (ed.), Important Early Synthetic Dyes. Chemistry, Constitution, Date, Properties. Conservation Analytical Laboratory, Smithsonian Institution, 1991.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3639
- Encyclopedia Britannica, http://www.britannica.com Comment: "Chemical Compound."(Accessed 12 May 2004).
- Website: www.probes.com/handbook/sections - gives absorption max=524 and emission max=544
- Website: member.pgonline.com/~bryand/dyes/45380.htm - absorption max = 516
- Colour Index International online at www.colour-index.org Comment: discoverer and date
- Sigma Dyes, Stains and Natural Pigments, Infrared Library, Nicolet, 1991-1995 Comment: OMNIC: formula= C20H8O5Br4, CAS= 548-26-5
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998