Difference between revisions of "Ultramarine blue, synthetic"
(Fixed internal links) |
|||
| (7 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:473 french ultramarine.jpg|thumb|French Blue]] | [[File:473 french ultramarine.jpg|thumb|French Blue]] | ||
== Description == | == Description == | ||
| − | + | [[File:Frultra C100x.jpg|thumb|French ultramarine at 100x (visible light left; UV light right)]] | |
| + | [[File:39_Ultramar.blue_synth_500X.jpg|thumb|Ultramarine blue, synthetic at 500s]] | ||
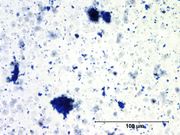
A pure, deep blue, fine particle, synthetic ultramarine discovered in 1826 in France by Jean-Baptiste Guimet and sold commercially in 1828. Synthetic ultramarine is prepared when anhydrous [[Sodium sulfate, anhydrous |sodium sulfate]] or [[sodium carbonate]] is mixed with clay, silica, sulfur, rosin and charcoal then slowly heated in a reducing atmosphere to 750º C (1,380º F). Variations in mixture proportions give various shades of blues, greens, violets and reds. Synthetic ultramarine blue has rounded particles that are finer and more regular in size and shape than the [[Ultramarine blue, natural |natural ultramarine]] pigment. The synthetic pigment is inexpensive and is used as a permanent artist pigment in oil and watercolor paints. Synthetic ultramarine is also used as a whitener in textiles and paper because in minimized yellowish shades. Additionally, it is used in [[ wallpaper]], [[soap]], textile printing, and [[laundry blue |laundry bluing]] agents. | A pure, deep blue, fine particle, synthetic ultramarine discovered in 1826 in France by Jean-Baptiste Guimet and sold commercially in 1828. Synthetic ultramarine is prepared when anhydrous [[Sodium sulfate, anhydrous |sodium sulfate]] or [[sodium carbonate]] is mixed with clay, silica, sulfur, rosin and charcoal then slowly heated in a reducing atmosphere to 750º C (1,380º F). Variations in mixture proportions give various shades of blues, greens, violets and reds. Synthetic ultramarine blue has rounded particles that are finer and more regular in size and shape than the [[Ultramarine blue, natural |natural ultramarine]] pigment. The synthetic pigment is inexpensive and is used as a permanent artist pigment in oil and watercolor paints. Synthetic ultramarine is also used as a whitener in textiles and paper because in minimized yellowish shades. Additionally, it is used in [[ wallpaper]], [[soap]], textile printing, and [[laundry blue |laundry bluing]] agents. | ||
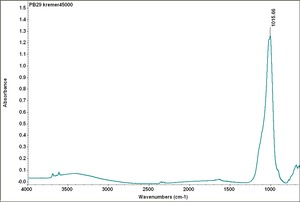
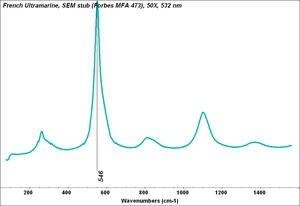
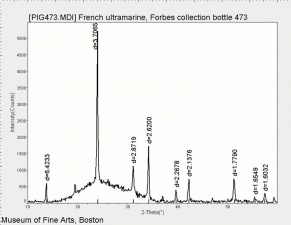
| − | [[ | + | [[[SliderGallery rightalign|PB29 kremer45000.TIF~FTIR (MFA)|French Ultramarine, SEM stub (Forbes MFA 473), 50X, 532 nm.TIF~Raman (MFA)|PIG473.jpg~XRD|f473sem.jpg~SEM|f473edsbw.jpg~EDS]]] |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
artificial ultramarine blue; Pigment Blue 29; CI 77007; French ultramarine; Ultramarinblau, synthetisch (Deut.); outremer de synthèse (Fr.); oyltramarina synthetiki (Gr.); blu oltremare artificiale (It.); blu Guimet (It.); ultramar (Esp.); ultramarijn (Ned.); ultramaryna (Pol.); ultramarijn blauw (syn) (Ned.); azul ultramarino, sintético (Port.); French blue; new blue; sky blue; Academy blue; permanent blue; Oriental blue; Gmelins blue; Guimet's blue; royal blue | artificial ultramarine blue; Pigment Blue 29; CI 77007; French ultramarine; Ultramarinblau, synthetisch (Deut.); outremer de synthèse (Fr.); oyltramarina synthetiki (Gr.); blu oltremare artificiale (It.); blu Guimet (It.); ultramar (Esp.); ultramarijn (Ned.); ultramaryna (Pol.); ultramarijn blauw (syn) (Ned.); azul ultramarino, sintético (Port.); French blue; new blue; sky blue; Academy blue; permanent blue; Oriental blue; Gmelins blue; Guimet's blue; royal blue | ||
| − | + | == Risks == | |
| − | |||
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | No significant hazards. Noncombustible. | + | * No significant hazards. |
| + | * Noncombustible. | ||
| − | == | + | ==Physical and Chemical Properties== |
| − | + | * Composition = 3Na2O.3Al2O3.6SiO2.2Na2S | |
| − | + | * Density = 2.34 g/ml | |
| + | * Refractive Index = 1.51; 1.63 | ||
| + | * Discolors when exposed to weak acids or sulfur fumes. | ||
| + | * Insoluble in water. | ||
| + | * Small, round uniform particles, no birefringence, no pleochroism, extinct in crossed polars. | ||
| + | * ASTM lightfastness=1 (excellent) | ||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_499.pdf|Characteristics of Common Blue Pigments]] |
| − | + | == Resources and Citations == | |
| − | |||
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| − | + | * J. Plesters, "Ultramarine Blue, Natural and Artificial", ''Artists Pigments'', Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. | |
| − | * ''Artists | + | * Pigments through the ages -http://webexhibits.org/pigments/indiv/technical/ultramarine.html |
| − | |||
| − | * | ||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | ||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| − | |||
* Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | ||
| − | |||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | |||
* E.J.LaBarre, ''Dictionary and Encyclopedia of Paper and Paper-making'', Swets & Zeitlinger, Amsterdam, 1969 | * E.J.LaBarre, ''Dictionary and Encyclopedia of Paper and Paper-making'', Swets & Zeitlinger, Amsterdam, 1969 | ||
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "ultramarine." Accessed 17 Mar. 2005 . | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "ultramarine." | ||
| − | |||
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | ||
| − | |||
* Colour Index International online at www.colour-index.org | * Colour Index International online at www.colour-index.org | ||
| − | |||
* Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 | ||
| − | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | |
| − | * Art and Architecture Thesaurus Online, | + | * Wikipedia: [https://en.wikipedia.org/wiki/Ultramarine Ultramarine] Accessed March 2025 |
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:08, 15 March 2025
Description
A pure, deep blue, fine particle, synthetic ultramarine discovered in 1826 in France by Jean-Baptiste Guimet and sold commercially in 1828. Synthetic ultramarine is prepared when anhydrous sodium sulfate or Sodium carbonate is mixed with clay, silica, sulfur, rosin and charcoal then slowly heated in a reducing atmosphere to 750º C (1,380º F). Variations in mixture proportions give various shades of blues, greens, violets and reds. Synthetic ultramarine blue has rounded particles that are finer and more regular in size and shape than the natural ultramarine pigment. The synthetic pigment is inexpensive and is used as a permanent artist pigment in oil and watercolor paints. Synthetic ultramarine is also used as a whitener in textiles and paper because in minimized yellowish shades. Additionally, it is used in Wallpaper, Soap, textile printing, and laundry bluing agents.
Synonyms and Related Terms
artificial ultramarine blue; Pigment Blue 29; CI 77007; French ultramarine; Ultramarinblau, synthetisch (Deut.); outremer de synthèse (Fr.); oyltramarina synthetiki (Gr.); blu oltremare artificiale (It.); blu Guimet (It.); ultramar (Esp.); ultramarijn (Ned.); ultramaryna (Pol.); ultramarijn blauw (syn) (Ned.); azul ultramarino, sintético (Port.); French blue; new blue; sky blue; Academy blue; permanent blue; Oriental blue; Gmelins blue; Guimet's blue; royal blue
Risks
- No significant hazards.
- Noncombustible.
Physical and Chemical Properties
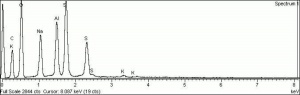
- Composition = 3Na2O.3Al2O3.6SiO2.2Na2S
- Density = 2.34 g/ml
- Refractive Index = 1.51; 1.63
- Discolors when exposed to weak acids or sulfur fumes.
- Insoluble in water.
- Small, round uniform particles, no birefringence, no pleochroism, extinct in crossed polars.
- ASTM lightfastness=1 (excellent)
Comparisons
Characteristics of Common Blue Pigments
Resources and Citations
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- J. Plesters, "Ultramarine Blue, Natural and Artificial", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993.
- Pigments through the ages -http://webexhibits.org/pigments/indiv/technical/ultramarine.html
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- E.J.LaBarre, Dictionary and Encyclopedia of Paper and Paper-making, Swets & Zeitlinger, Amsterdam, 1969
- Encyclopedia Britannica, http://www.britannica.com Comment: "ultramarine." Accessed 17 Mar. 2005 .
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Colour Index International online at www.colour-index.org
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Wikipedia: Ultramarine Accessed March 2025