Difference between revisions of "Acetal resin"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A thermoplastic polymer produced by the polymerization of formaldehyde into very long, linear oxymethylene (-O-CH2-O-CH2-) chains. Polyacetal was first produced in 1959 by DuPont under the name [[Delrin | + | A thermoplastic polymer produced by the polymerization of formaldehyde into very long, linear oxymethylene (-O-CH2-O-CH2-) chains. Polyacetal was first produced in 1959 by DuPont under the name [[Delrin|Delrin®]]. The highly crystalline resin is glossy, dense, stiff and strong. Among the toughest and most fatigue resistance of commercial thermoplastics, it is often used as a metal replacement. Acetal resin is resistant to moisture, heat, chemicals and solvents, but is sensitive to temperature. It can be metal plated, injection molded, welded, machined or extruded. Acetal resin is used for mechanical parts (gears, bushings), pipes, automotive parts, communication equipment, videocassettes, and cosmetic containers. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 11: | Line 11: | ||
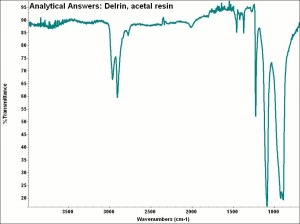
[[[SliderGallery rightalign|aaiDELRINacetal.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiDELRINacetal.jpg~FTIR]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | Combustible but very slow burning. Decomposes at high temperatures. May produce formaldehyde on decomposition. | ||
| + | == Physical and Chemical Properties == | ||
Soluble in dimethylformamide, benzyl alcohol. Insoluble in methanol, diethyl ether, aliphatic hydrocarbons. Burns soot-free. Resistant to acids, and alkalis. | Soluble in dimethylformamide, benzyl alcohol. Insoluble in methanol, diethyl ether, aliphatic hydrocarbons. Burns soot-free. Resistant to acids, and alkalis. | ||
| Line 20: | Line 23: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 165-175 | + | | 165-175 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.425 | + | | 1.425 g/ml |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_284.pdf|General Characteristics of Polymers]] |
| − | |||
| − | |||
| − | |||
| + | [[media:download_file_283.pdf|Physical Properties for Selected Thermoplastic Resins]] | ||
| − | == | + | == Resources and Citations == |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 791 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 791 | ||
| Line 50: | Line 47: | ||
* Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | ||
| − | * | + | * History of Plastics: www.nswpmith.com.au/historyofplastics.html |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 16:20, 18 April 2022
Description
A thermoplastic polymer produced by the polymerization of formaldehyde into very long, linear oxymethylene (-O-CH2-O-CH2-) chains. Polyacetal was first produced in 1959 by DuPont under the name Delrin®. The highly crystalline resin is glossy, dense, stiff and strong. Among the toughest and most fatigue resistance of commercial thermoplastics, it is often used as a metal replacement. Acetal resin is resistant to moisture, heat, chemicals and solvents, but is sensitive to temperature. It can be metal plated, injection molded, welded, machined or extruded. Acetal resin is used for mechanical parts (gears, bushings), pipes, automotive parts, communication equipment, videocassettes, and cosmetic containers.
Synonyms and Related Terms
AC; polyacetal; resina acetálica (Esp.); poliacetal (Esp.); acétal (Fr.); resina de acetal (Port.); polyoxymethylene; POM: polyoxide; polyether; polyformaldehyde
Examples: Delrin® [DuPont]; Celcon® [Ticona]; Hostaform® [Ticona]; Ultraform® [BASF];
Risks
Combustible but very slow burning. Decomposes at high temperatures. May produce formaldehyde on decomposition.
Physical and Chemical Properties
Soluble in dimethylformamide, benzyl alcohol. Insoluble in methanol, diethyl ether, aliphatic hydrocarbons. Burns soot-free. Resistant to acids, and alkalis.
TAP Plastics: Technical data sheet
| Melting Point | 165-175 C |
|---|---|
| Density | 1.425 g/ml |
Comparisons
General Characteristics of Polymers
Physical Properties for Selected Thermoplastic Resins
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 791
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- ASTM, Standard Terminology Relating to Thermophysical Properties, Annual Book of ASTM Standards, Section 6, Paints, Related Coatings and Aromatics, ASTM, E1142, 695-696, Jul-94
- M.Kaufman, The First Century of Plastics, The Plastics and Rubber Institute, London, 1963
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- History of Plastics: www.nswpmith.com.au/historyofplastics.html