Difference between revisions of "Ammonium sulfate"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (One intermediate revision by the same user not shown) | |||
| Line 9: | Line 9: | ||
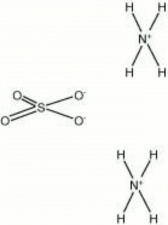
[[[SliderGallery rightalign|ammonium sulfate.jpg~Chemical structure]]] | [[[SliderGallery rightalign|ammonium sulfate.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | Nonflammable. May corrode metals. | ||
| + | |||
| + | Harmful if swallowed. Contact may cause irritation. | ||
| + | |||
| + | Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-a/S25176A.pdf SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in water (pH=5.5 at 0.1 mol/L). Insoluble in ethanol, acetone. | Soluble in water (pH=5.5 at 0.1 mol/L). Insoluble in ethanol, acetone. | ||
| Line 24: | Line 32: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 235-280 (dec) | + | | 235-280 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.77 | + | | 1.77 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 33: | Line 41: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 58 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 58 | ||
| Line 59: | Line 55: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 590 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 590 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Ammonium_sulfate (Accessed Jan. 25, 2006) |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Latest revision as of 13:37, 26 April 2022
Description
White or gray orthorhombic crystals that are used for flame proofing textiles and paper products, for tanning Leather, and for the manufacture of viscose Silk. Commercially, ammonium sulfate is also used as a fertilizer and as a reagent in water purification systems. Crystals of ammonium sulfate found in the bloom on painting varnishes and in a discolored area of a watercolor paper substrate may be due to Sulfur dioxide pollutants (Hatchfield 2002). In a closed environment, a saturated solution of ammonium sulfate will form an equilibrium at a relative humidity of about 81% (20C).
Synonyms and Related Terms
ammonium sulphate (Br.); svovlsur ammoniak (Dan.); Ammoniumsulfat (Deut., Sven.); diammonium sulfate; Actamaster; Dolamin; mascagnite
Risks
Nonflammable. May corrode metals.
Harmful if swallowed. Contact may cause irritation.
Fisher Scientific: SDS
Physical and Chemical Properties
Soluble in water (pH=5.5 at 0.1 mol/L). Insoluble in ethanol, acetone.
Deliquescent point at 20C is 80.6 % RH (see saturated salt solutions)
| Composition | (NH4)2SO4 |
|---|---|
| CAS | 7783-20-2 |
| Melting Point | 235-280 C (dec) |
| Density | 1.77 g/ml |
| Molecular Weight | mol. wt. = 132.14 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 58
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 590
- Wikipedia: http://en.wikipedia.org/wiki/Ammonium_sulfate (Accessed Jan. 25, 2006)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998