Difference between revisions of "Calcium sulfate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:Calcium sulfate hemihydrate.jpg|thumb|Calcium sulfate hemihydrate]] | ||
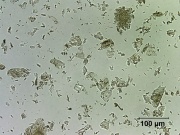
[[File:6_Calcium_sulfate_200X.jpg|thumb|Calcium sulfate]] | [[File:6_Calcium_sulfate_200X.jpg|thumb|Calcium sulfate]] | ||
== Description == | == Description == | ||
| − | + | [[File:6_Calcium_sulfate_200X_pol.jpg|thumb|Calcium sulfate]] | |
Commonly found in three forms: anhydrous ([[anhydrite]]), dihydrate ([[gypsum]]) and hemihydrate ([[plaster of Paris]]). Anhydrite is a colorless, inert pigment which is often used as a paper filler. Calcium sulfate dihydrate is used in the manufacture of portland cement. Gypsum is also used as a filler and pigment in paints, enamels, glazes, and paper. Plaster of Paris is used for wall plaster, wallboard, moldings, and statuary. | Commonly found in three forms: anhydrous ([[anhydrite]]), dihydrate ([[gypsum]]) and hemihydrate ([[plaster of Paris]]). Anhydrite is a colorless, inert pigment which is often used as a paper filler. Calcium sulfate dihydrate is used in the manufacture of portland cement. Gypsum is also used as a filler and pigment in paints, enamels, glazes, and paper. Plaster of Paris is used for wall plaster, wallboard, moldings, and statuary. | ||
| − | + | See more information on the pages for each form of hydration. | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | anhydrite; gypsum; plaster; terra alba; alabaster; calcium sulphate (Br.); mineral white; crown filler; alabastine; pearl filler | + | anhydrite; gypsum; plaster; terra alba; alabaster; calcium sulphate (Br.); mineral white; crown filler; alabastine; pearl filler; sulfate of lime |
| − | == | + | == Risks == |
| − | + | * Noncombustible | |
| − | + | ==Physical and Chemical Properties== | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | * Particle size = 0.2 micrometers |
| − | + | * Composition = CaSO4 (mol. wt. = 136.14) | |
| − | + | * CAS = 10101-41-4 | |
| + | * Appearance = white solid | ||
| + | * Odor = odorless | ||
== Comparisons == | == Comparisons == | ||
| Line 37: | Line 27: | ||
[[media:download_file_529.pdf|Characteristics of Common White Pigments]] | [[media:download_file_529.pdf|Characteristics of Common White Pigments]] | ||
| − | + | ==Resources and Citations== | |
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Calcium_sulfate Calcium sulfate] Accessed March 2025 | |
| − | == | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1753 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1753 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 1.21; 1.52; 1.53 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 1.21; 1.52; 1.53 | ||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:40, 12 March 2025
Description
Commonly found in three forms: anhydrous (Anhydrite), dihydrate (Gypsum) and hemihydrate (Plaster of Paris). Anhydrite is a colorless, inert pigment which is often used as a paper filler. Calcium sulfate dihydrate is used in the manufacture of portland cement. Gypsum is also used as a filler and pigment in paints, enamels, glazes, and paper. Plaster of Paris is used for wall plaster, wallboard, moldings, and statuary.
See more information on the pages for each form of hydration.
Synonyms and Related Terms
anhydrite; gypsum; plaster; terra alba; alabaster; calcium sulphate (Br.); mineral white; crown filler; alabastine; pearl filler; sulfate of lime
Risks
- Noncombustible
Physical and Chemical Properties
- Particle size = 0.2 micrometers
- Composition = CaSO4 (mol. wt. = 136.14)
- CAS = 10101-41-4
- Appearance = white solid
- Odor = odorless
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Wikipedia: Calcium sulfate Accessed March 2025
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1753
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 1.21; 1.52; 1.53