Difference between revisions of "Cobaltous arsenate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 7: | Line 7: | ||
cobalt violet light; Pigment Violet 14; CI 77350; erythrite (mineral); arseniato de cobalto (Esp.); arsenate de cobalt (Fr.); arseniato di cobalto (It.); arsenato de cobalto (Port.); cobalt arsenate; cobalt bloom; | cobalt violet light; Pigment Violet 14; CI 77350; erythrite (mineral); arseniato de cobalto (Esp.); arsenate de cobalt (Fr.); arseniato di cobalto (It.); arsenato de cobalto (Port.); cobalt arsenate; cobalt bloom; | ||
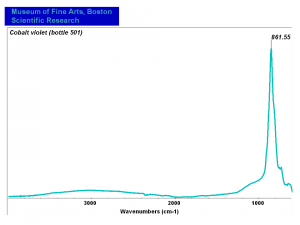
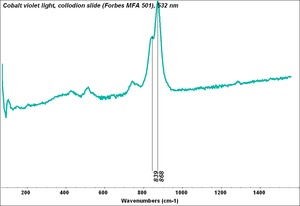
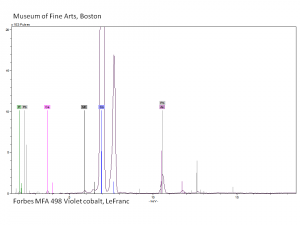
| + | [[[SliderGallery rightalign|Cobalt Violet(501).PNG~FTIR (MFA) (Forbes 501)|Cobalt violet light, collodion slide (Forbes MFA 501), 532 nm.TIF~Raman (MFA) (532nm)|Slide11_F498.PNG~XRF (MFA)]]] | ||
| − | == | + | == Risks == |
| − | + | Highly toxic by ingestion, inhalation, and skin contact. | |
| − | Prismatic or euhedral crystals; perfect cleavage parallel to long axes | + | == Physical and Chemical Properties == |
| + | |||
| + | * Soluble in dilute mineral acids and ammonium hydroxide. Insoluble in water. | ||
| + | * Prismatic or euhedral crystals; perfect cleavage parallel to long axes | ||
| + | * Weakly pleochroic | ||
| + | * High birefringence | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 20: | Line 26: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.06 | + | | 3.06 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 29: | Line 35: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 12:23, 30 May 2022
Description
A pale to medium violet pigment. Cobaltous arsenate, or light cobalt violet, occurs in nature as cobalt bloom or erythrite. Once it was synthetically produced in 1880, it became an important permanent, violet pigment. Cobaltous arsenate is now rarely used because of its toxicity. It has been replaced by the use of cobaltous phosphate and cobaltous ammonium phosphate. Cobaltous arsenate was used as a colorant in paints, glass, glazes, and enamels.
Synonyms and Related Terms
cobalt violet light; Pigment Violet 14; CI 77350; erythrite (mineral); arseniato de cobalto (Esp.); arsenate de cobalt (Fr.); arseniato di cobalto (It.); arsenato de cobalto (Port.); cobalt arsenate; cobalt bloom;
Risks
Highly toxic by ingestion, inhalation, and skin contact.
Physical and Chemical Properties
- Soluble in dilute mineral acids and ammonium hydroxide. Insoluble in water.
- Prismatic or euhedral crystals; perfect cleavage parallel to long axes
- Weakly pleochroic
- High birefringence
| Composition | Co3(AsO4)2 - 8H2O |
|---|---|
| Density | 3.06 g/ml |
| Molecular Weight | mol. wt. = 454.64 |
| Refractive Index | 1.626-1.701 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2495
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: prepared first in 1880