Difference between revisions of "Polyvinyl formal"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A [ | + | A [[thermoplastic|thermoplastic]] resin made by the condensation of [[polyvinyl alcohol]] with [[formaldehyde|formaldehyde]]. Polyvinyl formal is a colorless, flexible tough solid. It can be molded, extruded, or cast and is resistant to [[grease|greases]], [[oil|oils]], and [[alkali|alkalis]]. It is used as a structural adhesive for bonding aircraft components. |
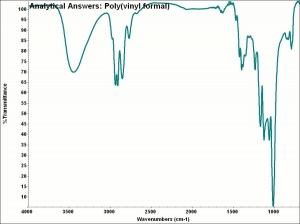
| − | + | [[[SliderGallery rightalign|aaiP_VINYLFORMAL.jpg~FTIR]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
poly(vinyl formal); polivinilformaldehido (Esp.); Formvar; polyvinyl acetal | poly(vinyl formal); polivinilformaldehido (Esp.); Formvar; polyvinyl acetal | ||
| − | + | ==Physical and Chemical Properties== | |
| − | |||
| − | == | ||
| − | |||
| − | |||
| − | + | * Soluble in glacial acetic acid, Cellosolve acetate, chloroform, diacetone alcohol, phenol, pyridine, THF, toluene. | |
| − | + | * Resistant to alkalis, greases and oils. | |
| − | + | * Density = 1.3 g/ml | |
| − | |||
| − | |||
| − | == | + | ==Resources and Citations== |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 848 |
| − | * | + | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Conservation Materials Ltd., Catalog |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:53, 27 September 2022
Description
A Thermoplastic resin made by the condensation of Polyvinyl alcohol with Formaldehyde. Polyvinyl formal is a colorless, flexible tough solid. It can be molded, extruded, or cast and is resistant to greases, oils, and alkalis. It is used as a structural adhesive for bonding aircraft components.
Synonyms and Related Terms
poly(vinyl formal); polivinilformaldehido (Esp.); Formvar; polyvinyl acetal
Physical and Chemical Properties
- Soluble in glacial acetic acid, Cellosolve acetate, chloroform, diacetone alcohol, phenol, pyridine, THF, toluene.
- Resistant to alkalis, greases and oils.
- Density = 1.3 g/ml
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 848
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Conservation Materials Ltd., Catalog