Difference between revisions of "Sulfamic acid"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White, crystalline solid. Sulfamic acid is used as a flame retardant for textiles and wood, buffer, acid cleaner, chlorine stabilizer in swimming pools, nitrite scavenger, and sulfonating agent. It is also used as a [ | + | White, crystalline solid. Sulfamic acid is used as a flame retardant for textiles and wood, buffer, acid cleaner, chlorine stabilizer in swimming pools, nitrite scavenger, and sulfonating agent. It is also used as a [[bleaching%20agent|bleaching agent]] for paper pulp, textiles, and color photographs. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
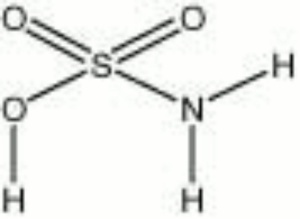
[[[SliderGallery rightalign|sulfamic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|sulfamic acid.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Toxic by ingestion. | ||
| + | * Corrosive to eyes, skin and lungs causing irritation and burns. | ||
| + | * Heating results in the production of toxic sulfur dioxide fumes. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=A295500&productDescription=SULFAMIC+ACID+CERTIFIED+500G&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in water, but hydrolyzed in water to form ammonium bisulfate. Slightly soluble in organic solvents. | Soluble in water, but hydrolyzed in water to form ammonium bisulfate. Slightly soluble in organic solvents. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 205 (dec) | + | | 205 C (dec) |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.15 | + | | 2.15 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 782 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 782 | ||
Latest revision as of 13:21, 6 June 2022
Description
White, crystalline solid. Sulfamic acid is used as a flame retardant for textiles and wood, buffer, acid cleaner, chlorine stabilizer in swimming pools, nitrite scavenger, and sulfonating agent. It is also used as a Bleaching agent for paper pulp, textiles, and color photographs.
Synonyms and Related Terms
amidosulfonic acid; amidosulfuric acid; sulfamidic acid
Risks
- Toxic by ingestion.
- Corrosive to eyes, skin and lungs causing irritation and burns.
- Heating results in the production of toxic sulfur dioxide fumes.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, but hydrolyzed in water to form ammonium bisulfate. Slightly soluble in organic solvents.
| Composition | SOOHNH2 |
|---|---|
| CAS | 5329-14-6 |
| Melting Point | 205 C (dec) |
| Density | 2.15 g/ml |
| Molecular Weight | mol. wt. = 97.1 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 782
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9090