Difference between revisions of "Strontium chromate"
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A pale yellow pigment that is also called strontium yellow. Strontium yellow was used in oil paints in the late 19th and early 20th centuries. It has good opacity and stability to light and heat. But because of its high hiding power, strontium chromate was not used in watercolors. Commercially strontium chromate was often mixed with other pigments; a mixture with [[Prussian%20blue|Prussian blue]] was sold as green cinnabar and a mixture with [[barium% | + | A pale yellow pigment that is also called strontium yellow. Strontium yellow was used in oil paints in the late 19th and early 20th centuries. It has good opacity and stability to light and heat. But because of its high hiding power, strontium chromate was not used in watercolors. Commercially strontium chromate was often mixed with other pigments; a mixture with [[Prussian%20blue|Prussian blue]] was sold as green cinnabar and a mixture with [[barium%20yellow|barium yellow]] and [[zinc%20yellow|zinc yellow]] was sold under the name lemon yellow or citron yellow. Currently strontium yellow is used in anticorrosive coating and pyrotechnics. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 8: | Line 8: | ||
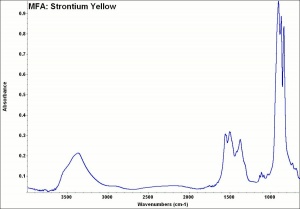
[[[SliderGallery rightalign|MFA- Strontium Yellow.jpg~FTIR]]] | [[[SliderGallery rightalign|MFA- Strontium Yellow.jpg~FTIR]]] | ||
| + | == Risks == | ||
| − | == | + | * Human carcinogen. |
| + | * Skin contact may cause allergies. Acute ingestion may cause fatal chromium poisoning. | ||
| + | * Chronic inhalation may cause lung cancer and respiratory irritation. | ||
| + | * May turn green is strong sunlight. | ||
| + | * American Elements: [file:///C:/Users/mderr/Downloads/strontium-chromate-7789-06-2_sds%20(1).pdf SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in dilute acids and hot water. Slightly soluble in cold water. | Soluble in dilute acids and hot water. Slightly soluble in cold water. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.89 | + | | 3.89 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * H. Kuhn, M.Curran, "Chrome Yellow and Other Chromate Pigments", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| Line 53: | Line 52: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9001 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9001 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:29, 6 June 2022
Description
A pale yellow pigment that is also called strontium yellow. Strontium yellow was used in oil paints in the late 19th and early 20th centuries. It has good opacity and stability to light and heat. But because of its high hiding power, strontium chromate was not used in watercolors. Commercially strontium chromate was often mixed with other pigments; a mixture with Prussian blue was sold as green cinnabar and a mixture with Barium yellow and Zinc yellow was sold under the name lemon yellow or citron yellow. Currently strontium yellow is used in anticorrosive coating and pyrotechnics.
Synonyms and Related Terms
lemon yellow; strontium yellow; Pigment Yellow 32; CI 77839; cromatpo de estroncio (Esp.); chromate de strontium (Fr.); cromato de estrôncio (Port.); strontium chrome; strontaine yellow; green cinnabar; lemon yellow; citron yellow
Risks
- Human carcinogen.
- Skin contact may cause allergies. Acute ingestion may cause fatal chromium poisoning.
- Chronic inhalation may cause lung cancer and respiratory irritation.
- May turn green is strong sunlight.
- American Elements: SDS
Physical and Chemical Properties
Soluble in dilute acids and hot water. Slightly soluble in cold water.
| Composition | SrCrO4 |
|---|---|
| CAS | 7789-06-2 |
| Density | 3.89 g/ml |
| Molecular Weight | mol. wt. = 203.62 |
| Refractive Index | 1.92; 2.01 |
Resources and Citations
- H. Kuhn, M.Curran, "Chrome Yellow and Other Chromate Pigments", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 610
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9001
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000