Difference between revisions of "Silicone resin"
| (8 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A [[polymer|polymer]] that contains [[silicon|silicon]], [[carbon|carbon]], and [[oxygen|oxygen]]. Silicone resins were first discovered by F. Kipping in England in 1904, but were not commercially produced until 1943 by Dow Corning. They were called silicones because their empirical formula (R2SiO) is similar to that for ketones (R2CO) (Lewis, 1993). Silicone resins are made by the room-temperature vulcanization ([[RTV|RTV]]) of [[silicone%20oil|silicone oils]]. They can cure either with moisture in the air (single-component system) or by the addition of a peroxide catalyst (two-component system). Once cured, silicone resins are chemically inert and can exist as [[elastomer|elastomers]] and [[synthetic%20resin|resins]] (both [[thermoset|thermoset]] and [[thermoplastic|thermoplastic]]). They function over a wide temperature range, are water repellent and have very poor adhesion. Silicones are used as [[sealant|sealants]], molding compounds, varnishes, enamels, gaskets, implants, and unbreakable windows. The use of silicone resins for preparing molds of museum objects is not recommended as residual curing compounds, release agents, and uncured silicone oils can stain porous materials and corrode metals. | + | A [[polymer|polymer]] that contains [[silicon|silicon]], [[carbon|carbon]], and [[oxygen|oxygen]]. Silicone resins were first discovered by F. Kipping in England in 1904, but were not commercially produced until 1943 by Dow Corning. They were called silicones because their empirical formula (R2SiO) is similar to that for ketones (R2CO) (Lewis, 1993). Silicone resins are made by the room-temperature vulcanization ([[RTV|RTV]]) of [[silicone%20oil|silicone oils]]. They can cure either with moisture in the air (single-component system) or by the addition of a peroxide catalyst (two-component system). Once cured, silicone resins are chemically inert and can exist as [[elastomer|elastomers]] and [[synthetic%20resin|resins]] (both [[thermoset|thermoset]] and [[thermoplastic|thermoplastic]]). They function over a wide temperature range, are water repellent and have very poor adhesion. Silicones are used as [[sealant|sealants]], molding compounds, varnishes, enamels, gaskets, implants, and unbreakable windows. The use of silicone resins for preparing molds of museum objects is not recommended as residual curing compounds, release agents, and uncured silicone oils can stain porous materials and corrode metals. For more information, [[Silicone cure systems]]. |
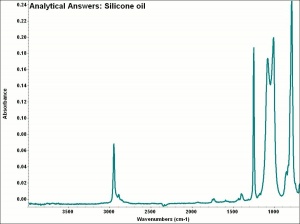
| − | + | [[[SliderGallery rightalign|aaiSILICNE.jpg~FTIR]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 10: | Line 10: | ||
Examples: Silastic [Dow]; Clear Museum Gel [Ready America] | Examples: Silastic [Dow]; Clear Museum Gel [Ready America] | ||
| − | == | + | == Risks == |
| − | Properties | + | * May contain [[acetone|acetone]] or [[methylene chloride|methylene chloride]] as a solvent. |
| + | * Single component silicone resins release [[acetic%20acid|acetic acid]] or [[methanol|methanol]] during cure. | ||
| + | * Silicone Solutions: [http://www.siliconesolutions.com/media/pdf/SS-5061SDS.pdf SDS for clear gel] | ||
| + | ==Physical and Chemical Properties== | ||
| − | Some silicone resins do not burn, others burn slightly with yellow flame and gray smoke. | + | * Properties include chemical inertness, thermal stability, low surface tension, and high compressibility. |
| − | + | * Some silicone resins do not burn, others burn slightly with yellow flame and gray smoke. | |
| − | Water repellent. Most are stable to about 300C. | + | * Water repellent. |
| − | + | * Most are stable to about 300C. | |
| − | + | * Density = 0.80 g/ml | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 40: | Line 29: | ||
[[media:download_file_299.pdf|Physical Properties for Selected Thermoset Resins]] | [[media:download_file_299.pdf|Physical Properties for Selected Thermoset Resins]] | ||
| + | ==Resources and Citations== | ||
| − | + | * J. P.Maish, "Silicone rubber staining of terracotta surfaces", ''Studies in Conservation'' 39:250–56, 1994. | |
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 719 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 719 | ||
| Line 56: | Line 45: | ||
* Thomas C. Jester (ed.), ''Twentieth-Century Building Materials'', McGraw-Hill Companies, Washington DC, 1995 | * Thomas C. Jester (ed.), ''Twentieth-Century Building Materials'', McGraw-Hill Companies, Washington DC, 1995 | ||
| − | * | + | * History of Plastics at www.nswpmith.com.au/historyofplastics.html |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 11:59, 13 September 2022
Description
A Polymer that contains Silicon, Carbon, and Oxygen. Silicone resins were first discovered by F. Kipping in England in 1904, but were not commercially produced until 1943 by Dow Corning. They were called silicones because their empirical formula (R2SiO) is similar to that for ketones (R2CO) (Lewis, 1993). Silicone resins are made by the room-temperature vulcanization (RTV) of silicone oils. They can cure either with moisture in the air (single-component system) or by the addition of a peroxide catalyst (two-component system). Once cured, silicone resins are chemically inert and can exist as elastomers and resins (both Thermoset and Thermoplastic). They function over a wide temperature range, are water repellent and have very poor adhesion. Silicones are used as sealants, molding compounds, varnishes, enamels, gaskets, implants, and unbreakable windows. The use of silicone resins for preparing molds of museum objects is not recommended as residual curing compounds, release agents, and uncured silicone oils can stain porous materials and corrode metals. For more information, Silicone cure systems.
Synonyms and Related Terms
SI; polysiloxane; resina de silicona (Esp.); résine silicone (Fr.); resina siliconica (It.); resina de silicone (Port.); organosiloxane; polymethyl siloxane; polydimethylsiloxane; RTV (room temperature vulcanization)
Examples: Silastic [Dow]; Clear Museum Gel [Ready America]
Risks
- May contain Acetone or Methylene chloride as a solvent.
- Single component silicone resins release Acetic acid or Methanol during cure.
- Silicone Solutions: SDS for clear gel
Physical and Chemical Properties
- Properties include chemical inertness, thermal stability, low surface tension, and high compressibility.
- Some silicone resins do not burn, others burn slightly with yellow flame and gray smoke.
- Water repellent.
- Most are stable to about 300C.
- Density = 0.80 g/ml
Comparisons
General Characteristics of Polymers
Physical Properties for Selected Thermoset Resins
Resources and Citations
- J. P.Maish, "Silicone rubber staining of terracotta surfaces", Studies in Conservation 39:250–56, 1994.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 719
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas C. Jester (ed.), Twentieth-Century Building Materials, McGraw-Hill Companies, Washington DC, 1995
- History of Plastics at www.nswpmith.com.au/historyofplastics.html