Difference between revisions of "Cellosolve"
Jump to navigation
Jump to search
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
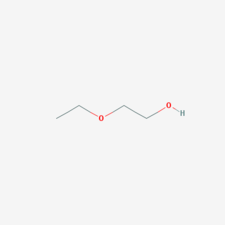
| − | [Union Carbide] A registered trademark name for the first in a series of "Cellosolve" solvents based on glycol ethers. Cellosolve is composed of [[ | + | [Union Carbide] A registered trademark name for the first in a series of "Cellosolve" solvents based on glycol ethers. Cellosolve is composed of [[Ethylene_glycol_monoethyl_ether|ethylene glycol monoethyl ether]]. This colorless, odorless liquid is miscible with both water and most organic solvents. Cellosolve dissolves many oils, resins, and waxes. It is used as a solvent for nitrocellulose resins, spray lacquers, varnishes, and enamels. Cellosolve is also used in varnish removers and dry cleaning solutions. Other applications include use in dye baths, leather finishing, and as an emulsifier. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 7: | Line 7: | ||
2-ethoxyethanol; ethylene glycol monoethyl ether; Cellusolve (sp); Oxitol | 2-ethoxyethanol; ethylene glycol monoethyl ether; Cellusolve (sp); Oxitol | ||
| − | [[[SliderGallery rightalign|Cellosolve. | + | [[[SliderGallery rightalign|Cellosolve.png~Chemical structure]]] |
| − | == | + | == Risks == |
| + | |||
| + | * Combustible. Flash point = 111F. | ||
| + | * Toxic by skin absorption. | ||
| + | * Inhalation and ingestion may cause irritation to membranes. | ||
| + | * Colonial Chemical: [https://www.chemistrystore.com/SDS/butyl%20cellosolve%20sds.pdf SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
Miscible with water, ethanol, ether, acetone, liquid esters. | Miscible with water, ethanol, ether, acetone, liquid esters. | ||
| Line 22: | Line 29: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | -70 | + | | -70 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.931 | + | | 0.931 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 38: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 135 | + | | 135 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Dow Chemical: [https://www.dow.com/en-us/product-technology/pt-solvents-glycols.html Solvents and Glycols] | |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Latest revision as of 08:53, 24 May 2022
Description
[Union Carbide] A registered trademark name for the first in a series of "Cellosolve" solvents based on glycol ethers. Cellosolve is composed of Ethylene glycol monoethyl ether. This colorless, odorless liquid is miscible with both water and most organic solvents. Cellosolve dissolves many oils, resins, and waxes. It is used as a solvent for nitrocellulose resins, spray lacquers, varnishes, and enamels. Cellosolve is also used in varnish removers and dry cleaning solutions. Other applications include use in dye baths, leather finishing, and as an emulsifier.
Synonyms and Related Terms
2-ethoxyethanol; ethylene glycol monoethyl ether; Cellusolve (sp); Oxitol
Risks
- Combustible. Flash point = 111F.
- Toxic by skin absorption.
- Inhalation and ingestion may cause irritation to membranes.
- Colonial Chemical: SDS
Physical and Chemical Properties
Miscible with water, ethanol, ether, acetone, liquid esters.
| Composition | CH2OH-CH2-O-C2H5 |
|---|---|
| CAS | 110-80-5 |
| Melting Point | -70 C |
| Density | 0.931 g/ml |
| Molecular Weight | mol. wt. = 90.12 |
| Boiling Point | 135 C |
Resources and Citations
- Dow Chemical: Solvents and Glycols
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 303
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Kurt Wehlte, The Materials and Techniques of Painting, Van Nostrand Reinhold Co., New York, 1975
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3797
- Website address 1 Comment: Molecular Probe - www.probes.com/probes/legal/html
- Website address 2 Comment: conservation termlist - www.hants.org.uk/museums