Difference between revisions of "Viridian"
| (5 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
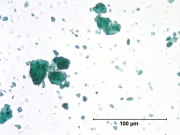
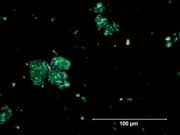
| − | [[File:Viridian C100x.jpg|thumb|Viridian]] | + | [[File:Viridian C100x.jpg|thumb|Viridian at 100x (visible light left; UV light right)]] |
== Description == | == Description == | ||
| − | + | [[File:37_Viridian_500X.jpg|thumb|Viridian at 500x]] | |
A permanent green pigment composed of hydrated chromium oxide. Viridian is a synthetic pigment with characteristic deep green, transparent particles which are unaffected by light and chemicals. The process for making the hydrated form of chromium oxide was discovered in 1838 by Pannetier in Paris. A less expensive method for manufacturing viridian was patented by Guignet in 1859. Viridian, though not extremely popular because of its high price, was used as a pigment in all types of binding media. The stable pigment is also used as a colorant in concrete mixtures, rubber, inks, and automotive paints. | A permanent green pigment composed of hydrated chromium oxide. Viridian is a synthetic pigment with characteristic deep green, transparent particles which are unaffected by light and chemicals. The process for making the hydrated form of chromium oxide was discovered in 1838 by Pannetier in Paris. A less expensive method for manufacturing viridian was patented by Guignet in 1859. Viridian, though not extremely popular because of its high price, was used as a pigment in all types of binding media. The stable pigment is also used as a colorant in concrete mixtures, rubber, inks, and automotive paints. | ||
| − | + | [[File:37_Viridian_500X_pol.jpg|thumb|Viridian at 500x polarized light]] | |
| − | [[File: | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
chromium oxide hydrate; Pigment Green 18; CI 77289; transparent chrome oxide; vert émeraude (Fr.); Chromoxidhydratgrün (Deut.); Veridian (Deut); viridiana (Esp.); verde smeraldo (It.); viridiaan (Ned.); viridian (Port.); chromium oxide green transparent; chromium oxide dihydrate; French Veronese green; Pannetier's green; Mittlers' green; Guignet's green; Casali's green | chromium oxide hydrate; Pigment Green 18; CI 77289; transparent chrome oxide; vert émeraude (Fr.); Chromoxidhydratgrün (Deut.); Veridian (Deut); viridiana (Esp.); verde smeraldo (It.); viridiaan (Ned.); viridian (Port.); chromium oxide green transparent; chromium oxide dihydrate; French Veronese green; Pannetier's green; Mittlers' green; Guignet's green; Casali's green | ||
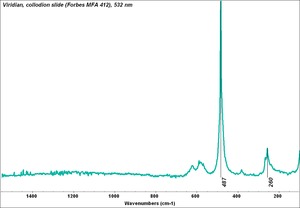
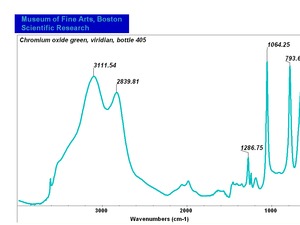
| − | [[[SliderGallery rightalign| | + | [[[SliderGallery rightalign|Viridian, collodion slide (Forbes MFA 412), 532 nm- resize.tif~Raman (MFA)|Viridian, chromium oxide green (bottle 405).TIF~FTIR (MFA)|f412sem.jpg~SEM|f412edsbw.jpg~EDS|viridian.jpg~Chemical structure]]] |
| − | + | ==Risks== | |
| − | == | + | * Gamblin Colors: [https://gamblincolors.com/wp-content/uploads/2016/03/SDSDryPigmentViridian.pdf SDS] |
| − | + | ==Physical and Chemical Properties== | |
| − | Particle size irregular (0.07 - 20 micrometers). | + | * Resistant to acids and alkalis. |
| + | * Particle size irregular (0.07 - 20 micrometers). | ||
| + | * Irregular undulose extinction in polarized light. | ||
| + | * Bright green birefringent particles. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 2435 | + | | 2435 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 3.32 | + | | 3.32 g/ml |
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| Line 33: | Line 35: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 4000 | + | | 4000 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | + | * R. Newman, "Chromium Oxide Greens", ''Artists Pigments'', Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | ||
| Line 61: | Line 47: | ||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | * | + | * Pigments Through the Ages - http://webexhibits.org/pigments/indiv/technical/viridian.html - refractive index: alpha and beta = 1.62; gamma = 2.12 |
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | ||
| Line 71: | Line 57: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Viridian (Accessed Sept. 20, 2005) |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 14:22, 25 June 2022
Description
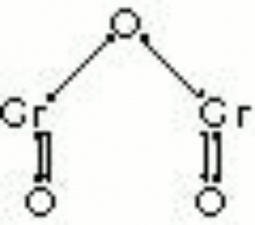
A permanent green pigment composed of hydrated chromium oxide. Viridian is a synthetic pigment with characteristic deep green, transparent particles which are unaffected by light and chemicals. The process for making the hydrated form of chromium oxide was discovered in 1838 by Pannetier in Paris. A less expensive method for manufacturing viridian was patented by Guignet in 1859. Viridian, though not extremely popular because of its high price, was used as a pigment in all types of binding media. The stable pigment is also used as a colorant in concrete mixtures, rubber, inks, and automotive paints.
Synonyms and Related Terms
chromium oxide hydrate; Pigment Green 18; CI 77289; transparent chrome oxide; vert émeraude (Fr.); Chromoxidhydratgrün (Deut.); Veridian (Deut); viridiana (Esp.); verde smeraldo (It.); viridiaan (Ned.); viridian (Port.); chromium oxide green transparent; chromium oxide dihydrate; French Veronese green; Pannetier's green; Mittlers' green; Guignet's green; Casali's green
Risks
- Gamblin Colors: SDS
Physical and Chemical Properties
- Resistant to acids and alkalis.
- Particle size irregular (0.07 - 20 micrometers).
- Irregular undulose extinction in polarized light.
- Bright green birefringent particles.
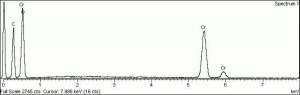
| Composition | Cr2O3.2H2O |
|---|---|
| CAS | 1308-38-9 |
| Melting Point | 2435 C |
| Density | 3.32 g/ml |
| Refractive Index | 1.62; 2.12 |
| Boiling Point | 4000 C |
Resources and Citations
- R. Newman, "Chromium Oxide Greens", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: p. 173
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Pigments Through the Ages - http://webexhibits.org/pigments/indiv/technical/viridian.html - refractive index: alpha and beta = 1.62; gamma = 2.12
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Wikipedia: http://en.wikipedia.org/wiki/Viridian (Accessed Sept. 20, 2005)