Difference between revisions of "Phthalocyanine blue"
| (14 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
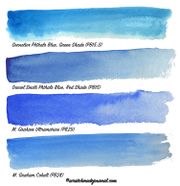
| + | [[File:blue phthalocyanines.jpg|thumb|Phthalo blue comparison<br>scratchmadejournal.com]] | ||
== Description == | == Description == | ||
A synthetic organic colorant composed of copper phthalocyanine that was first synthesized in 1933 by R.P.Linstead. In 1935, copper phthalocyanine was marketed as a paint pigment called [[Monastral blue]] [ICI]. The organic colorant is usually adsorbed on an aluminum hydrate base to form a deep blue color. Other colors are achieved by varying the formulation, i.e., chlorinated copper phthalocyanine produces a green colorant. Phthalocyanine blue is a permanent pigment which is unaffected by light, heat, and chemicals. It is used as a colorant in inks, enamels, plastics, paints, chalks, leather, pencils, and photographs. In industry, phthalocyanine blue has replaced all other blue pigments for use in coatings because it is lightfast as well as resistant to chemicals and clumping. | A synthetic organic colorant composed of copper phthalocyanine that was first synthesized in 1933 by R.P.Linstead. In 1935, copper phthalocyanine was marketed as a paint pigment called [[Monastral blue]] [ICI]. The organic colorant is usually adsorbed on an aluminum hydrate base to form a deep blue color. Other colors are achieved by varying the formulation, i.e., chlorinated copper phthalocyanine produces a green colorant. Phthalocyanine blue is a permanent pigment which is unaffected by light, heat, and chemicals. It is used as a colorant in inks, enamels, plastics, paints, chalks, leather, pencils, and photographs. In industry, phthalocyanine blue has replaced all other blue pigments for use in coatings because it is lightfast as well as resistant to chemicals and clumping. | ||
| − | + | == Types == | |
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{| class="wikitable" | {| class="wikitable" | ||
| + | ! Pigment number !! Manufacture !! Pigment name !! Manufacture CI number !! Comments | ||
|- | |- | ||
| − | + | | PB15:1 || Kremer || phthalo blue, primary|| 23050 || | |
| − | | | ||
|- | |- | ||
| − | + | | PB15:1 || Sun || phthalocyanine blue red shade|| 248-3745 || | |
| − | | | ||
|- | |- | ||
| − | + | | PB15:3 || Kremer || unspecified|| 23060 || | |
| − | | | + | |- |
| + | | PB15:3 || Sun || phthalocyanine green shade beta || 249-1282 || | ||
| + | |- | ||
| + | | PB15:3 || Magruder|| phthalocyanine blue g_s || bl1301-dc || | ||
|- | |- | ||
| − | |||
| − | |||
|} | |} | ||
| − | == | + | == Synonyms and Related Terms == |
| − | + | Pigment Blue 15; CI 74160; Heliogenblau (Deut.); bleu héliogène (Fr.); bleu de phtalocyanine (Fr.); Phtalocyaninblau (Deut.); azul de ftalocianina (Esp.); blu ftalo (It.); phtalocyanine blauw (Ned.); azul de ftalocianina (Port.); phthalo blue; copper phthalocyanine blue; Monastral Fast Blue; Winsor blue; Bocour blue; Talens Rembrandt blue; Thalo blue; Heliogen blue | |
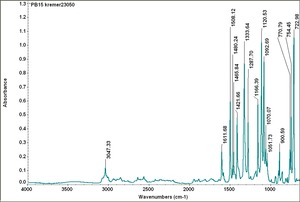
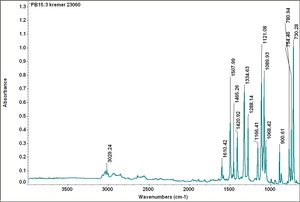
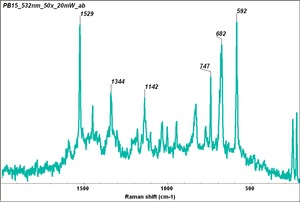
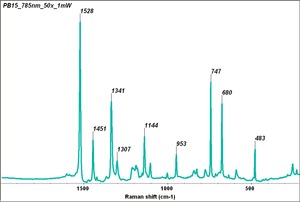
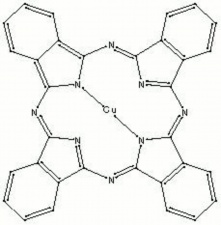
| − | Fisher Scientific: [https://fscimage.fishersci.com/msds/87778.htm MSDS] | + | [[[SliderGallery rightalign|PB15 kremer23050.TIF~FTIR 15 (MFA)|PB15-3 kremer 23060.TIF~FTIR 15:3(MFA)|PB15 532nm 50x 20mW ab.TIF~Raman (MFA) 532nm|PB15 785nm 50x 1mW.TIF~Raman (MFA) 785nm|phthalocyanine blue.jpg~Chemical structure]]] |
| + | |||
| + | == Risks == | ||
| + | |||
| + | * Suspected carcinogen, teratogen. | ||
| + | * May be contaminated with traces of PCB. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/87778.htm MSDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
| + | |||
| + | * Slowly turns yellow then dissolves in concentrated sulfuric acid | ||
| + | * Insoluble in organic solvents, water and alkalies. | ||
| + | * Fine grain translucent to opaque particles with moderate to strong birefringence | ||
| + | * Crystal type I appears deep red through Chelsea filter and crystal type II appears black | ||
| + | * Composition = C32H16N8Cu | ||
| + | * CAS = 147-14-8 | ||
| + | * Density = 1.6 | ||
| + | * Refractive Index = 1.38 | ||
== Comparisons == | == Comparisons == | ||
| Line 42: | Line 47: | ||
[[media:download_file_492.pdf|Characteristics of Common Blue Pigments]] | [[media:download_file_492.pdf|Characteristics of Common Blue Pigments]] | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | == | ||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| Line 54: | Line 57: | ||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: Phthalocyanine blue was put on the market in Britain in 1935 and in America in 1936. | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: Phthalocyanine blue was put on the market in Britain in 1935 and in America in 1936. | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | ||
| Line 62: | Line 65: | ||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | * Website | + | * Website: www.handprint.com - R. Linstead analyzed and named the molecule in 1933...it was commercially introduced in 1935 |
* Sigma Dyes, Stains and Natural Pigments, Infrared Library, Nicolet, 1991-1995 Comment: OMNIC: formula= C32H16N8Cu, CAS= 147-14-8 | * Sigma Dyes, Stains and Natural Pigments, Infrared Library, Nicolet, 1991-1995 Comment: OMNIC: formula= C32H16N8Cu, CAS= 147-14-8 | ||
Latest revision as of 11:41, 4 August 2022
Description
A synthetic organic colorant composed of copper phthalocyanine that was first synthesized in 1933 by R.P.Linstead. In 1935, copper phthalocyanine was marketed as a paint pigment called Monastral blue [ICI]. The organic colorant is usually adsorbed on an aluminum hydrate base to form a deep blue color. Other colors are achieved by varying the formulation, i.e., chlorinated copper phthalocyanine produces a green colorant. Phthalocyanine blue is a permanent pigment which is unaffected by light, heat, and chemicals. It is used as a colorant in inks, enamels, plastics, paints, chalks, leather, pencils, and photographs. In industry, phthalocyanine blue has replaced all other blue pigments for use in coatings because it is lightfast as well as resistant to chemicals and clumping.
Types
| Pigment number | Manufacture | Pigment name | Manufacture CI number | Comments |
|---|---|---|---|---|
| PB15:1 | Kremer | phthalo blue, primary | 23050 | |
| PB15:1 | Sun | phthalocyanine blue red shade | 248-3745 | |
| PB15:3 | Kremer | unspecified | 23060 | |
| PB15:3 | Sun | phthalocyanine green shade beta | 249-1282 | |
| PB15:3 | Magruder | phthalocyanine blue g_s | bl1301-dc |
Synonyms and Related Terms
Pigment Blue 15; CI 74160; Heliogenblau (Deut.); bleu héliogène (Fr.); bleu de phtalocyanine (Fr.); Phtalocyaninblau (Deut.); azul de ftalocianina (Esp.); blu ftalo (It.); phtalocyanine blauw (Ned.); azul de ftalocianina (Port.); phthalo blue; copper phthalocyanine blue; Monastral Fast Blue; Winsor blue; Bocour blue; Talens Rembrandt blue; Thalo blue; Heliogen blue
Risks
- Suspected carcinogen, teratogen.
- May be contaminated with traces of PCB.
- Fisher Scientific: MSDS
Physical and Chemical Properties
- Slowly turns yellow then dissolves in concentrated sulfuric acid
- Insoluble in organic solvents, water and alkalies.
- Fine grain translucent to opaque particles with moderate to strong birefringence
- Crystal type I appears deep red through Chelsea filter and crystal type II appears black
- Composition = C32H16N8Cu
- CAS = 147-14-8
- Density = 1.6
- Refractive Index = 1.38
Comparisons
Characteristics of Common Blue Pigments
Resources and Citations
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: ...was first introduced to the pigment trade under the name 'Monastral blue' at an exhibition in London, November 1935.
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: Phthalocyanine blue was put on the market in Britain in 1935 and in America in 1936.
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Website: www.handprint.com - R. Linstead analyzed and named the molecule in 1933...it was commercially introduced in 1935
- Sigma Dyes, Stains and Natural Pigments, Infrared Library, Nicolet, 1991-1995 Comment: OMNIC: formula= C32H16N8Cu, CAS= 147-14-8