Difference between revisions of "Ultramarine red"
Jump to navigation
Jump to search
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
[[File:46 ultramarine red.jpg|thumb|Ultramarine red]] | [[File:46 ultramarine red.jpg|thumb|Ultramarine red]] | ||
== Description == | == Description == | ||
| − | + | [[File:Ultramarinered C100x.jpg|thumb|Ultramarine red at 100x (visible light left; UV light right)]] | |
A rosy violet pigment that is a color variation produced in the manufacture of synthetic ultramarine blue. Ultramarine violets and reds were developed in Germany about 1875. The pigments are produced by treating ultramarine blue with sal ammoniac or dry hydrochloric acid gas at high temperatures. The sodium reacts to form sodium chloride, which is then washed away. Ultramarine red is a stable pigment but it has poor tinting strength and is rarely used in artists' paints (Plesters, 1993). | A rosy violet pigment that is a color variation produced in the manufacture of synthetic ultramarine blue. Ultramarine violets and reds were developed in Germany about 1875. The pigments are produced by treating ultramarine blue with sal ammoniac or dry hydrochloric acid gas at high temperatures. The sodium reacts to form sodium chloride, which is then washed away. Ultramarine red is a stable pigment but it has poor tinting strength and is rarely used in artists' paints (Plesters, 1993). | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
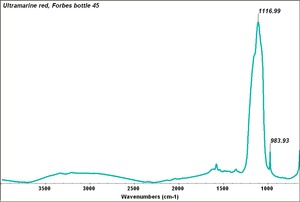
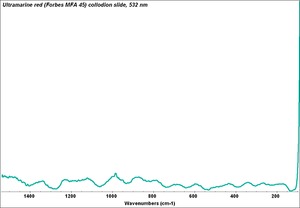
| − | + | [[[SliderGallery rightalign|Ultramarine red, 45.TIF~FTIR (MFA)|Ultramarine red (Forbes MFA 45) collodion slide, 532 nm copy.tif~Raman (MFA)]]] | |
ultramarine pink; ultramarine violet; Pigment Violet 15 | ultramarine pink; ultramarine violet; Pigment Violet 15 | ||
| − | + | == Risks == | |
| − | == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * No significant hazards. | |
| + | * Noncombustible. | ||
| − | + | ==Physical and Chemical Properties== | |
| − | == | + | * Discolors when exposed to weak acids or sulfur fumes. |
| + | * Insoluble in water. | ||
| + | * Fine, transparent pink grains. | ||
| + | * Isotropic. | ||
| + | * ASTM lightfastness=1 (excellent) | ||
| + | * Refractive Index = 1.56 | ||
| − | + | ==Resources and Citations== | |
| − | + | * J. Plesters, "Ultramarine Blue, Natural and Artificial", ''Artists Pigments'', Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. | |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Latest revision as of 08:58, 23 June 2022
Description
A rosy violet pigment that is a color variation produced in the manufacture of synthetic ultramarine blue. Ultramarine violets and reds were developed in Germany about 1875. The pigments are produced by treating ultramarine blue with sal ammoniac or dry hydrochloric acid gas at high temperatures. The sodium reacts to form sodium chloride, which is then washed away. Ultramarine red is a stable pigment but it has poor tinting strength and is rarely used in artists' paints (Plesters, 1993).
Synonyms and Related Terms
ultramarine pink; ultramarine violet; Pigment Violet 15
Risks
- No significant hazards.
- Noncombustible.
Physical and Chemical Properties
- Discolors when exposed to weak acids or sulfur fumes.
- Insoluble in water.
- Fine, transparent pink grains.
- Isotropic.
- ASTM lightfastness=1 (excellent)
- Refractive Index = 1.56
Resources and Citations
- J. Plesters, "Ultramarine Blue, Natural and Artificial", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"