Difference between revisions of "Purpurin"
Jump to navigation
Jump to search
| (One intermediate revision by one other user not shown) | |||
| Line 8: | Line 8: | ||
1,2,4-trihydroxyanthraquinone; Natural Red 16; CI 75410; purpurine; purpuriini (Fin.); purpurina (Esp., Port.) | 1,2,4-trihydroxyanthraquinone; Natural Red 16; CI 75410; purpurine; purpuriini (Fin.); purpurina (Esp., Port.) | ||
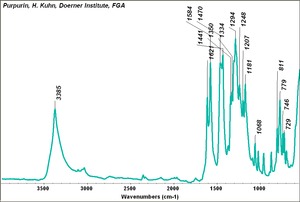
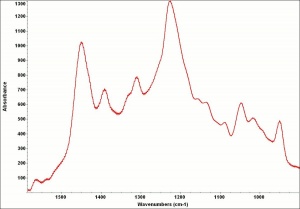
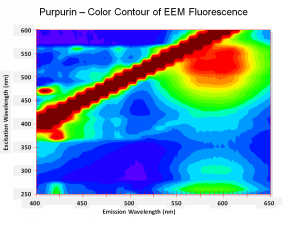
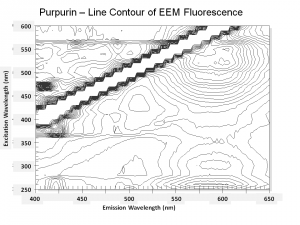
| − | [[[SliderGallery rightalign|Purpurin.TIF~FTIR (MFA)|PurpurinUCL.jpg~Raman|Purpurin color.PNG~EEM Color|Purpurin line.PNG~EEM Line]]] | + | [[[SliderGallery rightalign|Purpurin.TIF~FTIR (MFA)|PurpurinUCL.jpg~Raman (UCL)|Purpurin color.PNG~EEM Color|Purpurin line.PNG~EEM Line]]] |
| − | == | + | == Physical and Chemical Properties == |
| − | Soluble in alkali, benzene, ether, glacial acetic acid. Slightly soluble in hot water, ethanol. Fluoresces a bright yellow-red color. Bright red, needle crystals. Turns yellow in alkaline solutions. | + | * Soluble in alkali, benzene, ether, glacial acetic acid. |
| + | * Slightly soluble in hot water, ethanol. | ||
| + | * Fluoresces a bright yellow-red color. | ||
| + | * Bright red, needle crystals. Turns yellow in alkaline solutions. | ||
| + | * Melting Point = 263 | ||
| − | + | == Resources and Citations == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * H.Schweppe, J.Winter, "Madder and Alizarin", ''Artists Pigments'', Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. ° R. Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row, New York, 1969. | |
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 475 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 475 | ||
Latest revision as of 15:23, 15 August 2020
Description
One of the natural colorants extracted from Madder roots and other Rubiaceae family plants. Purpurin has been manufactured synthetically by the oxidation of alizarin since the 1920s. Purpurin forms a brilliant red color when mordanted with aluminum salts. It is not considered a permanent artists pigment (Mayer 1969). In red dyed fabric, the presence of purpurin along with alizarin has been used to distinguish natural madder dyes from the synthetic alizarin dyes first sold in 1871. Purpurin fluoresces a bright yellow-red while alizarin produces a pale violet color.
Synonyms and Related Terms
1,2,4-trihydroxyanthraquinone; Natural Red 16; CI 75410; purpurine; purpuriini (Fin.); purpurina (Esp., Port.)
Physical and Chemical Properties
- Soluble in alkali, benzene, ether, glacial acetic acid.
- Slightly soluble in hot water, ethanol.
- Fluoresces a bright yellow-red color.
- Bright red, needle crystals. Turns yellow in alkaline solutions.
- Melting Point = 263
Resources and Citations
- H.Schweppe, J.Winter, "Madder and Alizarin", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. ° R. Mayer, A Dictionary of Art Terms and Techniques, Harper and Row, New York, 1969.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 475
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Website address 1 Comment: http://www.coloria.net/varita.htm - Finnish names
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Artists' Pigments: A Handbook of their History and Characteristics, Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: H.Schweppe, J.Winter, "Madder and Alizarin"
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000