Difference between revisions of "Kevlar"
| (One intermediate revision by the same user not shown) | |||
| Line 12: | Line 12: | ||
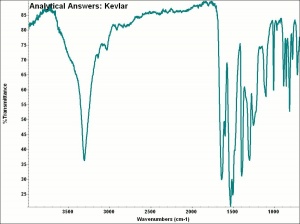
[[[SliderGallery rightalign|aaiKEVLAR.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiKEVLAR.jpg~FTIR]]] | ||
| − | == | + | ==Physical and Chemical Properties== |
| − | Resistant to organic solvents. Degrades in acid, alkalis and bleaches. Tenacity = 22 g/denier | + | * Resistant to organic solvents. |
| − | + | * Degrades in acid, alkalis and bleaches . | |
| − | + | * Tenacity = 22 g/denier | |
| − | + | * Moisture regain = ~7% | |
| − | + | * Elongation =~4% | |
| − | + | * Cross section = circular | |
| − | + | * Heat resistant to 400C without melting | |
| − | + | * Density = 1.44 g/ml | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 32: | Line 27: | ||
[[media:download_file_58.pdf|Properties of Synthetic Fibers]] | [[media:download_file_58.pdf|Properties of Synthetic Fibers]] | ||
| − | + | ==Resources and Citations== | |
| − | + | * DuPont: [https://www.dupont.com/brands/kevlar.html/ Kevlar Website] | |
| − | == | ||
| − | |||
* Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 | * Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 | ||
* Marjory L. Joseph, ''Introductory Textile Science'', Holt, Rinehart and Winston, Fort Worth, TX, 1986 | * Marjory L. Joseph, ''Introductory Textile Science'', Holt, Rinehart and Winston, Fort Worth, TX, 1986 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Kevlar (Accessed Nov. 9, 2005) |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | * | + | * Textile Worlds www.textileworld.com/categories/9905/fibers.html |
| − | * | + | * Smithsonian Institution: www.si.deu/lemelson/centerpieces/ilives/lexture05.html |
* Meredith Montague, contributed information, 1998 | * Meredith Montague, contributed information, 1998 | ||
| Line 54: | Line 47: | ||
* Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 | ||
| − | * Art and Architecture Thesaurus Online, | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:45, 22 September 2022
Description
[DuPont] A registered trademark for a para-aramid fiber (aromatic polyamides). Kevlar® fibers are composed of poly(p-phenylene terephthalamide), which is made from p-phenylene diamine and terephthaloyl chloride. Kevlar® was first made in 1965 by Stephanie Kwolek and Herbert Blades [DuPont] but not marketed until the 70s. It has five times the strength of a steel wire of the same diameter. Kevlar® also has excellent dielectric, thermal and mechanical properties and is resistant to fire, water, and biological growth. It is not resistant, however, to acids, alkalis and bleaches. Kevlar® is available as fibers, ropes, cables and fabric. It is widely used as a filler and reinforcing agent. Kevlar® is used in advanced composites for aircraft and aerospace applications. The extremely high strength and impact resistance of Kevlar® has led to its use in bullet proof vests. It is also used in tire cords, high pressure hoses, conveyer belts, cables, concrete reinforcement, race car bodies, sports equipment, and protective clothing.
See also Aramid fiber.
Synonyms and Related Terms
aramid; para-aramid;
Physical and Chemical Properties
- Resistant to organic solvents.
- Degrades in acid, alkalis and bleaches .
- Tenacity = 22 g/denier
- Moisture regain = ~7%
- Elongation =~4%
- Cross section = circular
- Heat resistant to 400C without melting
- Density = 1.44 g/ml
Comparisons
Properties of Synthetic Fibers
Resources and Citations
- DuPont: Kevlar Website
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- Marjory L. Joseph, Introductory Textile Science, Holt, Rinehart and Winston, Fort Worth, TX, 1986
- Wikipedia: http://en.wikipedia.org/wiki/Kevlar (Accessed Nov. 9, 2005)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Textile Worlds www.textileworld.com/categories/9905/fibers.html
- Smithsonian Institution: www.si.deu/lemelson/centerpieces/ilives/lexture05.html
- Meredith Montague, contributed information, 1998
- Product Information Comment: Manufacturer's literature
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000