Difference between revisions of "Glucose"
Jump to navigation
Jump to search
| (One intermediate revision by the same user not shown) | |||
| Line 9: | Line 9: | ||
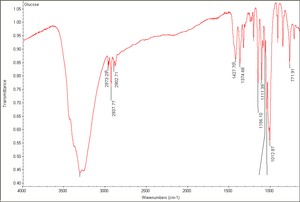
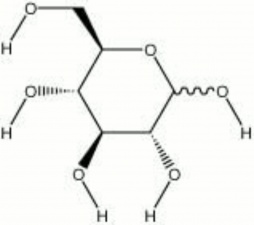
[[[SliderGallery rightalign|Glucose.TIF~FTIR (MFA)|glucose.jpg~Chemical structure]]] | [[[SliderGallery rightalign|Glucose.TIF~FTIR (MFA)|glucose.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Combustible. | ||
| + | * Incompatible with strong bases. | ||
| + | * Decomposition may produce toxic fumes. | ||
| + | * ThermoFisher: [https://www.fishersci.com/shop/msdsproxy?productName=AC410950010&productDescription=D(%252B)-GLUCOSE+ANHYDROUS+R+1KG&catNo=AC41095-0010&vendorId=VN00033901&storeId=10652 SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water. Slightly soluble in ethanol. | Soluble in water. Slightly soluble in ethanol. | ||
| Line 22: | Line 28: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 146-152 | + | | 146-152 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.544 | + | | 1.544 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 37: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 43: | Line 43: | ||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "glucose." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "glucose." Accessed: 9 Nov. 2004. |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 07:27, 30 August 2022
Description
A naturally occurring sugar found in plants and animals. Glucose is a clear, crystalline water-soluble carbohydrate most often used for flavoring foods and for fermentation. In art, aqueous solutions of glucose (Corn syrup) have been used as a Glycerol substitute to plasticize glue and starch pastes. Glucose can attract insects and is susceptible to biological growth.
Synonyms and Related Terms
sugar; dextrose, d-glucose; grape sugar; corn sugar; blood sugar; corn syrup
Risks
- Combustible.
- Incompatible with strong bases.
- Decomposition may produce toxic fumes.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water. Slightly soluble in ethanol.
| Composition | C6H12O6 |
|---|---|
| CAS | 50-99-7 |
| Melting Point | 146-152 C |
| Density | 1.544 g/ml |
| Molecular Weight | mol. wt. = 180.2 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Encyclopedia Britannica, http://www.britannica.com Comment: "glucose." Accessed: 9 Nov. 2004.
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998