Difference between revisions of "Red ocher"
Jump to navigation
Jump to search
| (4 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:Buffet198550.jpg|thumb|Buffet MFA#1989.50]] | + | [[File:Buffet198550.jpg|thumb|Buffet<br>MFA#1989.50]] |
[[File:90 red ocher.jpg|thumb|Red ocher]] | [[File:90 red ocher.jpg|thumb|Red ocher]] | ||
== Description == | == Description == | ||
| Line 5: | Line 5: | ||
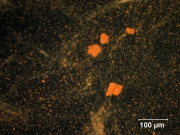
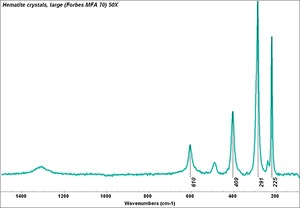
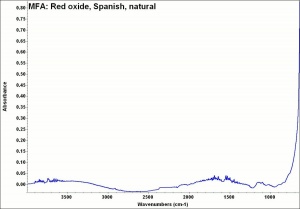
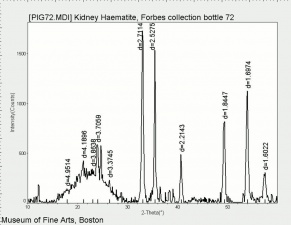
Any of several naturally occurring red earth pigments. Red ochers contain [[hematite|hematite]] ([[iron%20oxide%20red|red iron oxide]]) mixed with clay and vary widely in hue. Ground finely from red earth or clay, red ochers are lightfast, unreactive pigments that have been used since ancient times. They can also be manufactured by calcining [[yellow%20ocher|yellow ocher]] ([[goethite|goethite]]). | Any of several naturally occurring red earth pigments. Red ochers contain [[hematite|hematite]] ([[iron%20oxide%20red|red iron oxide]]) mixed with clay and vary widely in hue. Ground finely from red earth or clay, red ochers are lightfast, unreactive pigments that have been used since ancient times. They can also be manufactured by calcining [[yellow%20ocher|yellow ocher]] ([[goethite|goethite]]). | ||
| − | [[File:23_Red_ocher_200X.jpg|thumb|Red ocher]] | + | [[File:23_Red_ocher_200X.jpg|thumb|Red ocher at 200x]] |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
red ochre (Br.); red earth; earth red; iron oxide red; red iron oxide; Indian red; brun rouge; Roter Ocker (Deut.); minium de fer (Fr.); reddle; Spanish brown; Venetian red, English red; Spanish red; caput mortuum; Indian red; light red; burnt sienna | red ochre (Br.); red earth; earth red; iron oxide red; red iron oxide; Indian red; brun rouge; Roter Ocker (Deut.); minium de fer (Fr.); reddle; Spanish brown; Venetian red, English red; Spanish red; caput mortuum; Indian red; light red; burnt sienna | ||
| − | + | ==Physical and Chemical Properties == | |
| − | == | + | {| class="wikitable" |
| + | |- | ||
| + | ! scope="row"| Composition | ||
| + | | Fe2O3 | ||
| + | |- | ||
| + | ! scope="row"| Mohs Hardness | ||
| + | | 5.5 - 6.5 | ||
| + | |- | ||
| + | ! scope="row"| Density | ||
| + | | 4.2-5.3 g/ml | ||
| + | |- | ||
| + | ! scope="row"| Refractive Index | ||
| + | | 2.78; 3.01 | ||
| + | |} | ||
| − | + | [[[SliderGallery rightalign|Hematite crystals, large (Forbes MFA 70) 50X resize.tif~Raman (MFA)|MFA- Red oxide, Spanish, natural.jpg~FTIR|PIG72.jpg~XRD|fkhaemsem.jpg~SEM|fkhaemedsbw.jpg~EDS]]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| + | ==Resources and Citations== | ||
| + | * Ruth Siddall, 'Mineral Pigments in Archaeology: Their Analysis and the Range of Available Materials' ''Minerals'' Vol 8, p. 201 (2018). [https://www.academia.edu/36588315/Mineral_Pigments_in_Archaeology_Their_Analysis_and_the_Range_of_Available_Materials?email_work_card=view-paper Link] | ||
| + | * Helen Howard, Submitted information, November 2007 | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| − | |||
* Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | |||
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | + | * Pigments Through the Ages http://webexhibits.org/pigments/indiv/overview/redochre.html | |
| − | * | ||
| − | |||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" | ||
| + | Record content reviewed by EU-Artech, November 2007. | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:40, 28 February 2024
Description
Any of several naturally occurring red earth pigments. Red ochers contain Hematite (red iron oxide) mixed with clay and vary widely in hue. Ground finely from red earth or clay, red ochers are lightfast, unreactive pigments that have been used since ancient times. They can also be manufactured by calcining Yellow ocher (Goethite).
Synonyms and Related Terms
red ochre (Br.); red earth; earth red; iron oxide red; red iron oxide; Indian red; brun rouge; Roter Ocker (Deut.); minium de fer (Fr.); reddle; Spanish brown; Venetian red, English red; Spanish red; caput mortuum; Indian red; light red; burnt sienna
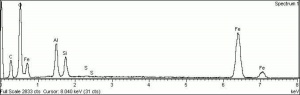
Physical and Chemical Properties
| Composition | Fe2O3 |
|---|---|
| Mohs Hardness | 5.5 - 6.5 |
| Density | 4.2-5.3 g/ml |
| Refractive Index | 2.78; 3.01 |
Resources and Citations
- Ruth Siddall, 'Mineral Pigments in Archaeology: Their Analysis and the Range of Available Materials' Minerals Vol 8, p. 201 (2018). Link
- Helen Howard, Submitted information, November 2007
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Pigments Through the Ages http://webexhibits.org/pigments/indiv/overview/redochre.html
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
Record content reviewed by EU-Artech, November 2007.