Difference between revisions of "Polylactic acid"
| (4 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
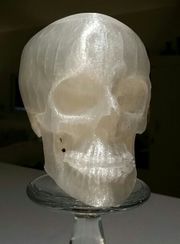
| − | A biodegradable a thermoplastic polyester made from renewable resources, such as beet, corn starch, cassava roots, or surgarcane. Polylactic acid was first developed in the 1980s in Japan. By 2010 it was the second most used bioplastic in the world. Polylactic acid is made by the polymerization of lactic acid and the ester lactide. The resultant polyester can be formed into fibers, sheets, and molded products. Early uses included absorbable sutures and dissolvable medical implants, but the product quickly expanded into the area of biodegradable consumer products such as cups, bottles, bags, and containers. It | + | A biodegradable a thermoplastic polyester made from renewable resources, such as beet, corn starch, cassava roots, or surgarcane. Polylactic acid was first developed in the 1980s in Japan. By 2010 it was the second most used bioplastic in the world. Polylactic acid is made by the polymerization of lactic acid and the ester lactide. The resultant polyester can be formed into fibers, sheets, and molded products. Early uses included absorbable sutures and dissolvable medical implants, but the product quickly expanded into the area of biodegradable consumer products such as cups, bottles, bags, and containers. It commonly used as feedstock 3d printing, either as the final product or as a mold that is later burnt out in a furnace. |
| + | * For polylactic acid fiber identification, see hhttp://cameo.mfa.org/wiki/Category:FRIL:_Polylactic_Acid_Fiber | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 10: | Line 11: | ||
== Applications == | == Applications == | ||
| − | == Risks == | + | == Personal and Collection Risks == |
AMPolymer.com: [https://web.archive.org/web/20090106174052/http://www.ampolymer.com/MSDS/PLA.pdf SDS] | AMPolymer.com: [https://web.archive.org/web/20090106174052/http://www.ampolymer.com/MSDS/PLA.pdf SDS] | ||
| + | |||
| + | == Environmental Risks == | ||
| + | |||
| + | PLA is biodegradable but can also be recycled, either chemically or mechanically. It is used as an alternative to ABS (in 3D printing), cellophane (in packaging) and shellac (as a biologically derived coating). | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| Line 30: | Line 35: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.210-1.430 | + | | 1.210-1.430 g/ml |
|} | |} | ||
| − | |||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 12:34, 22 October 2022
Description
A biodegradable a thermoplastic polyester made from renewable resources, such as beet, corn starch, cassava roots, or surgarcane. Polylactic acid was first developed in the 1980s in Japan. By 2010 it was the second most used bioplastic in the world. Polylactic acid is made by the polymerization of lactic acid and the ester lactide. The resultant polyester can be formed into fibers, sheets, and molded products. Early uses included absorbable sutures and dissolvable medical implants, but the product quickly expanded into the area of biodegradable consumer products such as cups, bottles, bags, and containers. It commonly used as feedstock 3d printing, either as the final product or as a mold that is later burnt out in a furnace.
- For polylactic acid fiber identification, see hhttp://cameo.mfa.org/wiki/Category:FRIL:_Polylactic_Acid_Fiber
Synonyms and Related Terms
Poly(lactic) acid; PLA; polylactide
Applications
Personal and Collection Risks
AMPolymer.com: SDS
Environmental Risks
PLA is biodegradable but can also be recycled, either chemically or mechanically. It is used as an alternative to ABS (in 3D printing), cellophane (in packaging) and shellac (as a biologically derived coating).
Physical and Chemical Properties
Soluble in chlorinated solvents, hot benzene, tetrahydrofuran and dioxane. Insoluble in water.
| CAS | 26100-51-6 |
|---|---|
| Melting Point | 130-180 C |
| Glass transition | 60-65 C |
| Density | 1.210-1.430 g/ml |