Difference between revisions of "Talc"
| (5 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | [[File:2000.1293-SC116389.jpg|thumb|]] | + | [[File:2000.1293-SC116389.jpg|thumb|Soapstone sculpture<br>MFA# 2001.218]] |
== Description == | == Description == | ||
A soft, slippery mineral composed of hydrated magnesium silicate. Talc is found in veins and masses in metamorphic rocks; large deposits occur in Austria (Tyrol), Italy (Florence), Switzerland, Germany (Bavaria), England (Cornwall), Scotland (Shetland), Canada (Quebec, Ontario), and the U.S. (New England, New York, Pennsylvania, Maryland, North Carolina, Texas). Talc, mixed with other minor minerals, occurs in the stone called [[steatite]], or soapstone. It is usually a white, green or gray color. Talc is so soft, it can be scratched with a fingernail. It has a waxy luster and a greasy feel. Steatite was used in antiquity for small carved objects, such as cylinder seals, scarabs, amulets, bowls, boxes, beads, and statuary. After carving, some pieces were baked to dehydrate the talc and thus harden the stone. Currently, talc is often crushed and used as a filler in paper, ceramics, paint, plastics, rubber, soaps, plaster, crayons, and cosmetics. It has also been used as a lubricant, leather dressing, dusting powder, and as an insulating material. Talc is used on the inside of powdered gloves and can leave residual powder on contacted surfaces. Since soapstones are resistant to most chemical reagents, and to moderate heat, they have been used for sinks and countertops. | A soft, slippery mineral composed of hydrated magnesium silicate. Talc is found in veins and masses in metamorphic rocks; large deposits occur in Austria (Tyrol), Italy (Florence), Switzerland, Germany (Bavaria), England (Cornwall), Scotland (Shetland), Canada (Quebec, Ontario), and the U.S. (New England, New York, Pennsylvania, Maryland, North Carolina, Texas). Talc, mixed with other minor minerals, occurs in the stone called [[steatite]], or soapstone. It is usually a white, green or gray color. Talc is so soft, it can be scratched with a fingernail. It has a waxy luster and a greasy feel. Steatite was used in antiquity for small carved objects, such as cylinder seals, scarabs, amulets, bowls, boxes, beads, and statuary. After carving, some pieces were baked to dehydrate the talc and thus harden the stone. Currently, talc is often crushed and used as a filler in paper, ceramics, paint, plastics, rubber, soaps, plaster, crayons, and cosmetics. It has also been used as a lubricant, leather dressing, dusting powder, and as an insulating material. Talc is used on the inside of powdered gloves and can leave residual powder on contacted surfaces. Since soapstones are resistant to most chemical reagents, and to moderate heat, they have been used for sinks and countertops. | ||
| − | |||
[[File:Talcemr3.jpg|thumb|Talc]] | [[File:Talcemr3.jpg|thumb|Talc]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 8: | ||
hydrated magnesium silicate; Pigment White 26; talc (Fr.); talk (Ned., Sven., Pol.); talco (Esp., It., Port.); Talk (Deut.); Talkum (Deut.); talkis (Gr.); talcum; soapstone; steatite; asbestine; French chalk; tailors chalk; Mistron®; Vertal; Nicron®; | hydrated magnesium silicate; Pigment White 26; talc (Fr.); talk (Ned., Sven., Pol.); talco (Esp., It., Port.); Talk (Deut.); Talkum (Deut.); talkis (Gr.); talcum; soapstone; steatite; asbestine; French chalk; tailors chalk; Mistron®; Vertal; Nicron®; | ||
[[File:soapstonelarge.jpg|thumb|Talc micrograph]] | [[File:soapstonelarge.jpg|thumb|Talc micrograph]] | ||
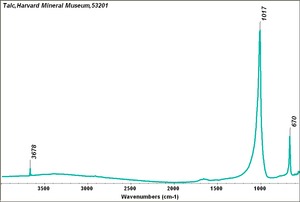
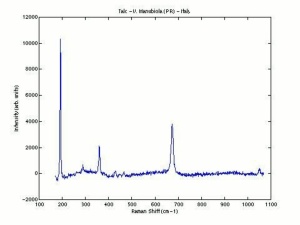
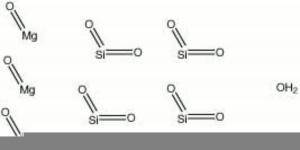
| − | [[[SliderGallery rightalign|Talc,Harvard Mineral Museum,53201.TIF~FTIR (MFA)|Talcitaly1.jpg~Raman|talc.jpg~Chemical structure]]] | + | [[[SliderGallery rightalign|Talc,Harvard Mineral Museum,53201.TIF~FTIR (MFA)|Talcitaly1.jpg~Raman(U of Parma)|talc.jpg~Chemical structure]]] |
== Risks == | == Risks == | ||
| − | Noncombustible. Some samples may contain asbestos. | + | * Noncombustible. |
| + | * Some samples may contain asbestos. | ||
| + | * Talc USA: [http://www.talcusa.com/wp-content/uploads/2015/11/TALC-USA-8020-MSDS-01202015.pdf MSDS] | ||
| − | |||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| − | + | * Composition = Mg3Si4O10(OH)2 | |
| − | Ground particles can be very small (2.0 micrometers). | + | * CAS = 14807-96-6 |
| − | + | * Mohs Hardness = 1.0 | |
| − | Talc occurs naturally in foliated masses | + | * Density = 2.5-2.8 g/ml |
| − | + | * Refractive Index = 1.539; 1.589; 1.589 | |
| − | + | * Birefringence = 0.050 - 0.051 | |
| − | + | * Insoluble in water, cold acids or alkalis | |
| − | + | * Ground particles can be very small (2.0 micrometers). | |
| − | + | * Talc occurs naturally in foliated to fibrous masses | |
| − | + | * Perfect cleavage in one direction producing thin laminar particles | |
| − | + | * Fracture = uneven | |
| − | + | * Luster = pearly, greasy | |
| − | + | * Streak = white to pearl black | |
| − | + | * Fluorescence = inert to weak pink or yellowish in LW; inert to pale orange in SW | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 55: | Line 40: | ||
* Mineralogy Database: [http://www.webmineral.com/data/Talc.shtml Talc] | * Mineralogy Database: [http://www.webmineral.com/data/Talc.shtml Talc] | ||
| − | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | |
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 793 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 793 | ||
| − | |||
* ''Encyclopedia Britannica'', http://www.britannica.com Comment: "talc" [Accessed March 4, 2002]. | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "talc" [Accessed March 4, 2002]. | ||
| − | |||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Talc Talc] (Accessed Sept. 17, 2005 and Dec 2022) | |
| − | * Wikipedia: | ||
| − | |||
* ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | * ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | |||
* Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| − | |||
* Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9207 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9207 | ||
| − | + | * Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | |
| − | * Art and Architecture Thesaurus Online, | ||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.7-2.8 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.7-2.8 | ||
| − | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:08, 12 March 2025
Description
A soft, slippery mineral composed of hydrated magnesium silicate. Talc is found in veins and masses in metamorphic rocks; large deposits occur in Austria (Tyrol), Italy (Florence), Switzerland, Germany (Bavaria), England (Cornwall), Scotland (Shetland), Canada (Quebec, Ontario), and the U.S. (New England, New York, Pennsylvania, Maryland, North Carolina, Texas). Talc, mixed with other minor minerals, occurs in the stone called Steatite, or soapstone. It is usually a white, green or gray color. Talc is so soft, it can be scratched with a fingernail. It has a waxy luster and a greasy feel. Steatite was used in antiquity for small carved objects, such as cylinder seals, scarabs, amulets, bowls, boxes, beads, and statuary. After carving, some pieces were baked to dehydrate the talc and thus harden the stone. Currently, talc is often crushed and used as a filler in paper, ceramics, paint, plastics, rubber, soaps, plaster, crayons, and cosmetics. It has also been used as a lubricant, leather dressing, dusting powder, and as an insulating material. Talc is used on the inside of powdered gloves and can leave residual powder on contacted surfaces. Since soapstones are resistant to most chemical reagents, and to moderate heat, they have been used for sinks and countertops.
Synonyms and Related Terms
hydrated magnesium silicate; Pigment White 26; talc (Fr.); talk (Ned., Sven., Pol.); talco (Esp., It., Port.); Talk (Deut.); Talkum (Deut.); talkis (Gr.); talcum; soapstone; steatite; asbestine; French chalk; tailors chalk; Mistron®; Vertal; Nicron®;
Risks
- Noncombustible.
- Some samples may contain asbestos.
- Talc USA: MSDS
Physical and Chemical Properties
- Composition = Mg3Si4O10(OH)2
- CAS = 14807-96-6
- Mohs Hardness = 1.0
- Density = 2.5-2.8 g/ml
- Refractive Index = 1.539; 1.589; 1.589
- Birefringence = 0.050 - 0.051
- Insoluble in water, cold acids or alkalis
- Ground particles can be very small (2.0 micrometers).
- Talc occurs naturally in foliated to fibrous masses
- Perfect cleavage in one direction producing thin laminar particles
- Fracture = uneven
- Luster = pearly, greasy
- Streak = white to pearl black
- Fluorescence = inert to weak pink or yellowish in LW; inert to pale orange in SW
Comparisons
Properties of Common Abrasives
Characteristics of Common White Pigments
Resources and Citations
- Mineralogy Database: Talc
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 793
- Encyclopedia Britannica, http://www.britannica.com Comment: "talc" [Accessed March 4, 2002].
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: Talc (Accessed Sept. 17, 2005 and Dec 2022)
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9207
- Art and Architecture Thesaurus Online, https://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.7-2.8