Difference between revisions of "Lead oxychloride"
m (→Description: removed image link) |
m (→Description) |
||
| (20 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:1979.808.13 coffeepot.JPG|thumb|Coffeepot, star motif in center contains lead oxychloride (Colonial Williamsburg Foundation 1979.808.13)]] | ||
| + | [[File:Patent Yellow Samuel Gore.JPG|thumb|Patent Yellow Advertisement, Continental Journal, June 3, 1784, Boston]] | ||
== Description == | == Description == | ||
| − | A strong yellow pigment | + | A strong yellow pigment known as Patent yellow, also called Turner's yellow, Montpelier Yellow, or Mineral Yellow. The pigment is reported various sources as both lead chloride oxide and lead oxychloride. |
| + | It was discovered in 1770 by chemist Karl Scheele (of Scheele's Green) who inadvertently produced it as a by-product during experiments with soda preparation. His work was not made public until 1775, when his finding was announced in a lecture by his friend and colleague, the Swedish chemist and naturalist Torbern Bergman. Although not invented by him, the technique for preparing the pigment was patented in 1781 by James Turner in England. Turner's yellow was made by mixing powdered litharge with saltwater, pouring off the alkali, then calcining the solid mass until the desired yellow was formed. This yellow pigment has a short window of use, from its introduction until the early 19th century, when it was eventually superseded by chrome yellow. | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | + | Possible lead oxychloride (Pb7O6Cl2); Reported in Eastaugh as patent yellow (PbCl2.5-7PbO); Montpelier yellow; Kassel yellow; mineral yellow; Vernona yellow; Cassel yellow (PbCl2-7PbO); Turner's yellow | |
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
! scope="row"| Composition | ! scope="row"| Composition | ||
| − | | PbCl2.5-7PbO | + | | PbCl2.5-7PbO and/or Pb7O6Cl2 |
|} | |} | ||
| − | == | + | == Risks == |
| − | + | * Reportedly turns black when exposed to water, UV light and sulfur fumes. | |
| + | * In some examples where this pigment was identified, the paint color had the appearance of a dull brownish-yellow ochre, similar to a tarnished bronze paint without the metallic glint. | ||
| − | == | + | ==Microscopic Characteristics== |
| + | |||
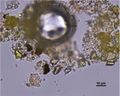
| + | In PPL, particles have a pale to medium lemon-yellow color with moderate to high relief. The particle size distribution is typically very coarse, with larger particles measuring as large as 15 microns. Smaller particles can appear shard-like, sometimes with conchoidal fracture, while larger particles can appear somewhat platy with a distinctive shallow stair-step pattern along some edges. RI > 1.662. | ||
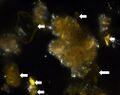
| + | In XPL, this pigment is reported (Eastaugh 693) as being highly birefringent with third and fourth order colors observed, but many particles have low first order birefringence, or appear dark with a yellow birefringence along the grain boundaries. Distinctively, some particles may show blue/violet interference colors. | ||
| + | |||
| + | <gallery> | ||
| + | File:Yellow PPL 1000x.JPG|thumb|lead oxychloride, PPL, 1000x | ||
| + | File:Yellow XPL 1000x.JPG|thumb|lead oxychloride showing anomalous violet-blues, XPL, 1000x | ||
| + | File:Drum patent yellow with arrows PPL 1000x.jpg|thumb|Patent Yellow in Dispersion, PPL, 1000x | ||
| + | File:Drum patent yellow with arrows XPL 1000x.jpg|thumb|Patent Yellow in dispersion, XPL, 1000x | ||
| + | </gallery> | ||
| + | |||
| + | == Resources and Citations == | ||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| Line 30: | Line 46: | ||
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" (gives date of 1781 for patent) | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" (gives date of 1781 for patent) | ||
| + | |||
| + | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 13:55, 21 December 2022
Description
A strong yellow pigment known as Patent yellow, also called Turner's yellow, Montpelier Yellow, or Mineral Yellow. The pigment is reported various sources as both lead chloride oxide and lead oxychloride. It was discovered in 1770 by chemist Karl Scheele (of Scheele's Green) who inadvertently produced it as a by-product during experiments with soda preparation. His work was not made public until 1775, when his finding was announced in a lecture by his friend and colleague, the Swedish chemist and naturalist Torbern Bergman. Although not invented by him, the technique for preparing the pigment was patented in 1781 by James Turner in England. Turner's yellow was made by mixing powdered litharge with saltwater, pouring off the alkali, then calcining the solid mass until the desired yellow was formed. This yellow pigment has a short window of use, from its introduction until the early 19th century, when it was eventually superseded by chrome yellow.
Synonyms and Related Terms
Possible lead oxychloride (Pb7O6Cl2); Reported in Eastaugh as patent yellow (PbCl2.5-7PbO); Montpelier yellow; Kassel yellow; mineral yellow; Vernona yellow; Cassel yellow (PbCl2-7PbO); Turner's yellow
| Composition | PbCl2.5-7PbO and/or Pb7O6Cl2 |
|---|
Risks
- Reportedly turns black when exposed to water, UV light and sulfur fumes.
- In some examples where this pigment was identified, the paint color had the appearance of a dull brownish-yellow ochre, similar to a tarnished bronze paint without the metallic glint.
Microscopic Characteristics
In PPL, particles have a pale to medium lemon-yellow color with moderate to high relief. The particle size distribution is typically very coarse, with larger particles measuring as large as 15 microns. Smaller particles can appear shard-like, sometimes with conchoidal fracture, while larger particles can appear somewhat platy with a distinctive shallow stair-step pattern along some edges. RI > 1.662. In XPL, this pigment is reported (Eastaugh 693) as being highly birefringent with third and fourth order colors observed, but many particles have low first order birefringence, or appear dark with a yellow birefringence along the grain boundaries. Distinctively, some particles may show blue/violet interference colors.
Resources and Citations
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- R. Newman, E. Farrell, 'House Paint Pigments', Paint in America , R. Moss ed., Preservation Press, New York City, 1994
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: first prepared in 1775
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments" (gives date of 1781 for patent)
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004