Difference between revisions of "Calcium hydroxyapatite"
Jump to navigation
Jump to search
| (One intermediate revision by the same user not shown) | |||
| Line 7: | Line 7: | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | tribasic calcium phosphate; tricalcium orthophosphate; tricalcium phosphate; bone ash | + | tribasic calcium phosphate; tricalcium orthophosphate; tricalcium phosphate; bone ash; hydroxylapatite |
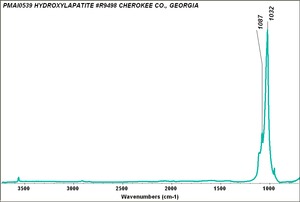
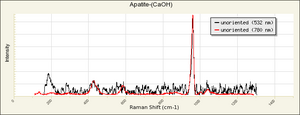
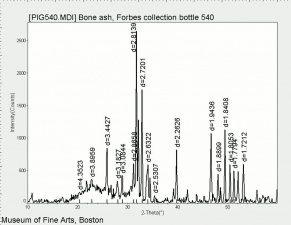
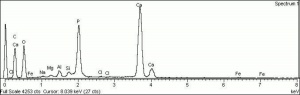
| − | [[[SliderGallery rightalign|Hydroxyapatite PMA.TIF~FTIR (PMA)|PIG540.jpg~XRD|f540sem.jpg~SEM|f540edsbw.jpg~EDS]]] | + | [[[SliderGallery rightalign|Hydroxyapatite PMA.TIF~FTIR (PMA)|Hydroxyapatite.png~Raman (RRUFF)|PIG540.jpg~XRD|f540sem.jpg~SEM|f540edsbw.jpg~EDS]]] |
== Risks == | == Risks == | ||
Latest revision as of 13:06, 2 December 2022
Description
A white, odorless, tasteless powder. Bone is primarily composed of calcium hydroxyapatite mixed with Calcium carbonate and organic binders. Calcium hydroxyapatite is used in the manufacture of Milk glass, as a polishing powder, as a mordant and as a buffer.
See also Apatite.
Synonyms and Related Terms
tribasic calcium phosphate; tricalcium orthophosphate; tricalcium phosphate; bone ash; hydroxylapatite
Risks
- Nonflammable.
- Merz USA: SDS
Physical and Chemical Properties
Soluble in mineral acids. Insoluble in water, ethanol, acetic acid.
| Composition | Ca10(PO4)6(OH)2 |
|---|---|
| CAS | 1306-06-5 |
| Melting Point | 1670 C |
| Density | 3.18 g/ml |
| Molecular Weight | mol. wt. = 1004.69 |
| Refractive Index | 1.63 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1741