Difference between revisions of "Chrome green"
Jump to navigation
Jump to search
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[File:350 chrome green.jpg|thumb|Chrome green]] | [[File:350 chrome green.jpg|thumb|Chrome green]] | ||
== Description == | == Description == | ||
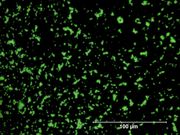
| − | + | [[File:chromegreen C100x.jpg|thumb|Chrome green at 100x (Visible light on left / UV light on right)]] | |
| + | [[File:32_Chrome_green_500X.jpg|thumb|Chrome green at 500x]] | ||
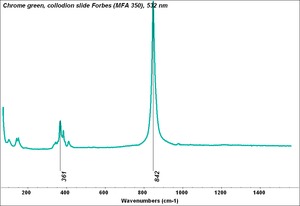
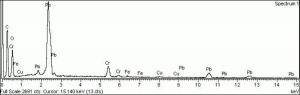
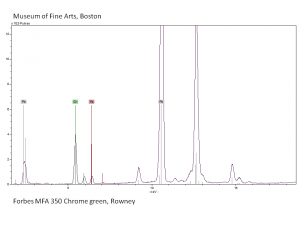
A pigment mixture prepared with chrome yellow ([[lead chromate]]) and [[Prussian blue]]. Chrome green has been used since the early 19th century, primarily in house paints and industrial products. It is not used in artists paints. | A pigment mixture prepared with chrome yellow ([[lead chromate]]) and [[Prussian blue]]. Chrome green has been used since the early 19th century, primarily in house paints and industrial products. It is not used in artists paints. | ||
| Line 31: | Line 32: | ||
| ~2.4 | | ~2.4 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Resources and Citations== | ==Resources and Citations== | ||
Latest revision as of 13:17, 29 May 2022
Description
A pigment mixture prepared with chrome yellow (Lead chromate) and Prussian blue. Chrome green has been used since the early 19th century, primarily in house paints and industrial products. It is not used in artists paints.
Synonyms and Related Terms
Pigment Green 15; CI 77600; verde cromo (Esp.); vert de chrome (Fr.); Zinnobergrün (Deut.); Chromoxydgrün (Deut.); prasino toy chromioy (Gr.); verde cromo (It.); chromaatgroen (Ned.); verde de crómio (Port.); cinnabar green; green vermilion; Victoria green; Prussian green; bronze green; Milori green; Brunswick green; nitrate green; royal green; zinnober green; oil green;
Risks
- Toxic by inhalation, ingestion and skin absorption.
- Human carcinogen and teratogen.
- Suspected mutagen.
- Solomon Colors: SDS
Physical and Chemical Properties
- Turns blue with exposure to strong light or acids.
- Turns dark orange with exposure to alkalis.
- Individual blue and yellow particles are small and cannot usually be distinguish microscopically.
| Density | 4.06 g/ml |
|---|---|
| Refractive Index | ~2.4 |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density=4.06, ref index=~2.4
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 611
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"