Difference between revisions of "Sulfur trioxide"
Jump to navigation
Jump to search
| (One intermediate revision by the same user not shown) | |||
| Line 2: | Line 2: | ||
An unstable solid that can sublime at room temperature. Sulfur trioxide is one of the sulfur oxide pollutants formed when [[sulfur|sulfur]] compounds are burned. It reacts readily and exothermically with water to form [[sulfuric%20acid|sulfuric acid]]. Sulfur trioxide is a strong oxidizing agent. It is primarily used industrially in [[nonionic%20detergent|nonionic detergents]], explosives, and solar energy collectors. | An unstable solid that can sublime at room temperature. Sulfur trioxide is one of the sulfur oxide pollutants formed when [[sulfur|sulfur]] compounds are burned. It reacts readily and exothermically with water to form [[sulfuric%20acid|sulfuric acid]]. Sulfur trioxide is a strong oxidizing agent. It is primarily used industrially in [[nonionic%20detergent|nonionic detergents]], explosives, and solar energy collectors. | ||
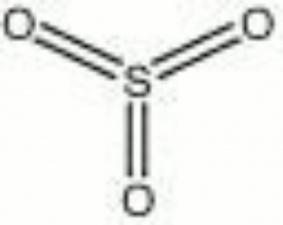
| + | [[[SliderGallery rightalign|sulfur trioxide.jpg~Chemical structure]]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 37: | Line 38: | ||
| 44.8 C | | 44.8 C | ||
|} | |} | ||
| − | |||
| − | |||
==Resources and Citations== | ==Resources and Citations== | ||
Latest revision as of 09:18, 7 June 2022
Description
An unstable solid that can sublime at room temperature. Sulfur trioxide is one of the sulfur oxide pollutants formed when Sulfur compounds are burned. It reacts readily and exothermically with water to form Sulfuric acid. Sulfur trioxide is a strong oxidizing agent. It is primarily used industrially in nonionic detergents, explosives, and solar energy collectors.
Synonyms and Related Terms
sulfuric anhydride; sulphur trioxide (Br.)
Risks
- Fire hazard in contact with flammable organic compounds.
- Highly toxic.
- Hygroscopic.
- Corrosive.
- Contact causes irritation and burns.
- Fisher Scientific: MSDS
Physical and Chemical Properties
| Composition | SO3 |
|---|---|
| CAS | 7446-11-9 |
| Melting Point | 16.8 C |
| Density | 1.9224 g/ml |
| Molecular Weight | mol. wt. = 80.06 |
| Boiling Point | 44.8 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9152
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982