Difference between revisions of "Gamma benzene hexachloride"
Jump to navigation
Jump to search
| Line 3: | Line 3: | ||
An odorless, white crystalline powder that is most commonly used as an [[insecticide]] and fumigant for the control of chiggers, ticks, fleas, cockroaches, lice, and ''Acarus scabiei''. The legal name for gamma benzene hexachloride is [[lindane]] and it is sold commercially as Gammexane. | An odorless, white crystalline powder that is most commonly used as an [[insecticide]] and fumigant for the control of chiggers, ticks, fleas, cockroaches, lice, and ''Acarus scabiei''. The legal name for gamma benzene hexachloride is [[lindane]] and it is sold commercially as Gammexane. | ||
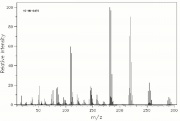
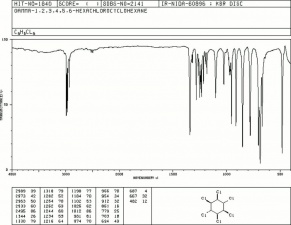
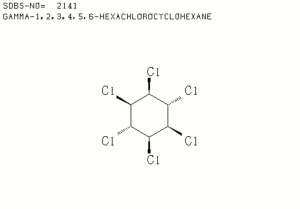
| − | + | [[[SliderGallery rightalign|lindaneIR.jpg~FTIR|lindanestruct.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 35: | Line 35: | ||
| 323 C | | 323 C | ||
|} | |} | ||
| − | |||
| − | |||
==Resources and Citations== | ==Resources and Citations== | ||
Latest revision as of 12:39, 25 July 2022
Description
An odorless, white crystalline powder that is most commonly used as an Insecticide and fumigant for the control of chiggers, ticks, fleas, cockroaches, lice, and Acarus scabiei. The legal name for gamma benzene hexachloride is Lindane and it is sold commercially as Gammexane.
Synonyms and Related Terms
BHC; lindane; benzene hexachloride; hexachlorocyclohexane; Gammexane [ICI]; Hexafor; Dolmix; Kotol; Soprocide; Kilbeech; Hexyclan
Risks
- Toxic by inhalation, ingestion and skin absorption. LD50 = 88-270 mg/kg.
- Potential carcinogen.
- NIH: Tech sheet
Physical and Chemical Properties
| Composition | C6H6Cl6 |
|---|---|
| CAS | 58-89-9 (608-73-1) |
| Melting Point | 113 C |
| Density | 1.87 g/ml |
| Molecular Weight | mol. wt.=290.8 |
| Boiling Point | 323 C |
Resources and Citations
- Nancy Odegaard, Alyce Sadongei, and associates, Old Poisons, New Problems, Altimira, Walnut Creek, CA, 2005
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- G.Caneva, M.P.Nugari, O.Salvadori, Biology in the Conservation of Works of Art, ICCROM, Rome, 1991
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976