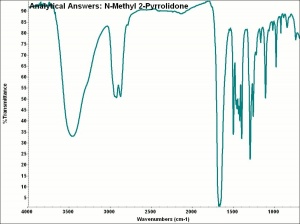
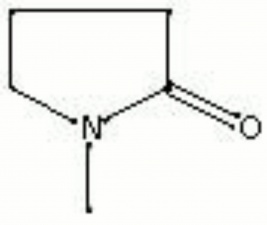
Difference between revisions of "Methylpyrrolidone"
Jump to navigation
Jump to search
(→Risks) |
|||
| Line 17: | Line 17: | ||
* Contact cause severe irritation to eyes and skin. | * Contact cause severe irritation to eyes and skin. | ||
* ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=O36884&productDescription=1-METHYL-2-PYRROLIDINONE+P+4L&vendorId=VN00033897&countryCode=US&language=en SDS] | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=O36884&productDescription=1-METHYL-2-PYRROLIDINONE+P+4L&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | * EPA lists methylpyrrolidone as hazardous waste (ignitable, toxic); Concentrations over 10% must be disposed of appropriately | ||
| + | |||
==Physical and Chemical Properties== | ==Physical and Chemical Properties== | ||
Latest revision as of 12:29, 17 April 2024
Description
A colorless liquid with a mild, amine-like odor. Methylpyrrolidone, or MP, is industrially as a
Solvent for the extraction of aromatic compounds from lubrication oils. It also dissolves many
polymers and old
varnishes.
Synonyms and Related Terms
methyl pyrrolidone; 1-methyl-2-pyrrolidinone; n-methyl-2-pyrrolidone; n-methyl-2-butyrolactone; NMP; MP; M-Pyrol
Risks
- Combustible. Flash point = 96C
- Decomposes on burning to produce toxic fumes including nitrogen oxides and carbon monoxide.
- Contact cause severe irritation to eyes and skin.
- ThermoFisher: SDS
- EPA lists methylpyrrolidone as hazardous waste (ignitable, toxic); Concentrations over 10% must be disposed of appropriately
Physical and Chemical Properties
Miscible with water, alcohols, ether, acetone, ethyl acetate, chloroform, benzene and castor oil.
| Composition | C5H9NO |
|---|---|
| CAS | 872-50-4 |
| Melting Point | -24.4 C |
| Density | 1.027 g/ml |
| Molecular Weight | mol. wt. = 99.1 |
| Refractive Index | 1.4690 |
| Boiling Point | 202 C |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 6197
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993