Difference between revisions of "Sapphire"
| (3 intermediate revisions by the same user not shown) | |||
| Line 12: | Line 12: | ||
* Insoluble in acids and alkalis. | * Insoluble in acids and alkalis. | ||
* Trigonal crystal system. | * Trigonal crystal system. | ||
| − | |||
* Fracture = conchoidal or splintery | * Fracture = conchoidal or splintery | ||
| − | * Luster = vitreous | + | * Luster = vitreous to subadamantine |
* Streak = white | * Streak = white | ||
| + | * Fluorescence = generally inert. Might fluoresce red to orange under LW. Weak chalky blue or green under SW may indicate heat treatment | ||
| + | * Pleochrosism = moderate to strong blue-violet to blue-green dichroism | ||
| + | * Inclusions = may have fine needle-like rutile inclusions; hexagonal growth or color banding; or twinning planes | ||
{| class="wikitable" | {| class="wikitable" | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
! scope="row"| Mohs Hardness | ! scope="row"| Mohs Hardness | ||
| 9.0 | | 9.0 | ||
| − | |||
| − | |||
| − | |||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| Line 35: | Line 28: | ||
|- | |- | ||
! scope="row"| Refractive Index | ! scope="row"| Refractive Index | ||
| − | | 1. | + | | 1.762 - 1.770 |
|- | |- | ||
! scope="row"| Birefringence | ! scope="row"| Birefringence | ||
| Line 55: | Line 48: | ||
==Resources and Citations== | ==Resources and Citations== | ||
| − | + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 693 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 693 | ||
| − | |||
* R.M.Organ, ''Design for Scientific Conservation of Antiquities'', Smithsonian Institution, Washington DC, 1968 | * R.M.Organ, ''Design for Scientific Conservation of Antiquities'', Smithsonian Institution, Washington DC, 1968 | ||
| − | |||
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
| − | |||
* R.F.Symmes, T.T.Harding, Paul Taylor, ''Rocks, Fossils and Gems'', DK Publishing, Inc., New York City, 1997 | * R.F.Symmes, T.T.Harding, Paul Taylor, ''Rocks, Fossils and Gems'', DK Publishing, Inc., New York City, 1997 | ||
| − | |||
* A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 Comment: 1200-500 BC | * A.Lucas, J.R.Harris, ''Ancient Egyptian Materials and Industries'', Edward Arnold Publishers Ltd., London, 4th edition, 1962 Comment: 1200-500 BC | ||
| − | |||
* ''Encyclopedia Britannica'', http://www.britannica.com Comment: "sapphire." Accessed 15 Sept. 2005. | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "sapphire." Accessed 15 Sept. 2005. | ||
| − | + | * Wikipedia: [https://en.wikipedia.org/wiki/Sapphire Sapphire] (Accessed Sept. 14, 2005 and Dec 2022) | |
| − | * Wikipedia: | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Latest revision as of 10:47, 4 January 2023
Description
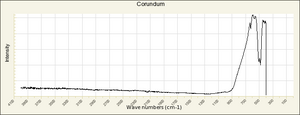
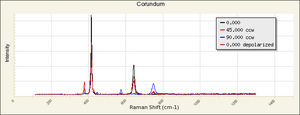
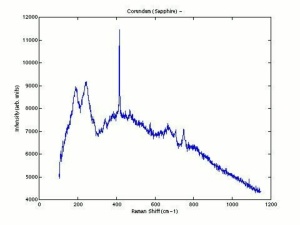
A transparent blue Gemstone composed of Corundum (aluminum oxide). Sapphires range in color from a pale blue to a deep indigo. They are mined in Myanmar (formerly Burma), Thailand, Kashmir, Sri Lanka, Australia (Victoria, Queensland, New South Wales), India, Madagascar, Russia, South Africa, and the U.S. (Montana, North Carolina). Sapphires are extremely hard and durable gemstones that have been used in jewelry since 1200 BCE. Oriented rutile crystal inclusions in a sapphire can produce a six-sided star effect called a Star Sapphire. Synthetic sapphires, produced commercially since 1902, are used in jewelry, watches, phonograph needles, instrument bearings, optical elements, and as abrasives.
Synonyms and Related Terms
corundum; alumina; aluminum oxide; star sapphire; safir (Dan.; Sven.); Saphir (Deut.); zafiro (Esp.); saphir (Fr.); saffier (Ned.); szafir (Pol.); safira (Port.);
Physical and Chemical Properties
- Insoluble in acids and alkalis.
- Trigonal crystal system.
- Fracture = conchoidal or splintery
- Luster = vitreous to subadamantine
- Streak = white
- Fluorescence = generally inert. Might fluoresce red to orange under LW. Weak chalky blue or green under SW may indicate heat treatment
- Pleochrosism = moderate to strong blue-violet to blue-green dichroism
- Inclusions = may have fine needle-like rutile inclusions; hexagonal growth or color banding; or twinning planes
| Mohs Hardness | 9.0 |
|---|---|
| Density | 3.96-4.05 g/ml |
| Refractive Index | 1.762 - 1.770 |
| Birefringence | 0.008-0.010 |
Comparisons
Properties of Common Abrasives
Properties of Common Gemstones
Additional Images
Resources and Citations
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 693
- R.M.Organ, Design for Scientific Conservation of Antiquities, Smithsonian Institution, Washington DC, 1968
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- R.F.Symmes, T.T.Harding, Paul Taylor, Rocks, Fossils and Gems, DK Publishing, Inc., New York City, 1997
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962 Comment: 1200-500 BC
- Encyclopedia Britannica, http://www.britannica.com Comment: "sapphire." Accessed 15 Sept. 2005.
- Wikipedia: Sapphire (Accessed Sept. 14, 2005 and Dec 2022)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976