Difference between revisions of "Acetal"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A colorless, volatile liquid obtained from the reaction of [http://cameo.mfa.org/materials/fullrecord.asp?name=acetaldehyde acetaldehyde] and [http://cameo.mfa.org/materials/fullrecord.asp?name=ethyl | + | A colorless, volatile liquid obtained from the reaction of [http://cameo.mfa.org/materials/fullrecord.asp?name=acetaldehyde acetaldehyde] and [http://cameo.mfa.org/materials/fullrecord.asp?name=ethyl%20alcohol ethyl alcohol]. Acetal has a pleasing odor and a nutlike aftertaste. It is used as a solvent, and as an ingredient in cosmetics, perfumes (such as jasmine) and flavorings. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 47: | Line 47: | ||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Website address 1 Comment: Fisher Scientific at https://www1.fishersci.com/catalogs/acrosgroup.jsp?catalogParamId=8013176&catalogParamType=AG Flash point = -21C |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:24, 24 July 2013
Description
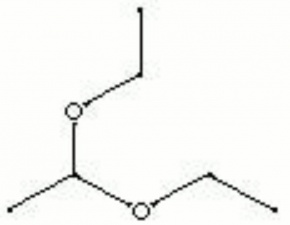
A colorless, volatile liquid obtained from the reaction of acetaldehyde and ethyl alcohol. Acetal has a pleasing odor and a nutlike aftertaste. It is used as a solvent, and as an ingredient in cosmetics, perfumes (such as jasmine) and flavorings.
Synonyms and Related Terms
diethylacetal; 1,1-diethoxyethane; ethylidenediethyl ether; acetaldehyde diethyl acetal
Other Properties
Soluble in hexane, ethanol and ethyl acetate. Slightly soluble in water.
| Composition | CH3CH(OC2H5)2 |
|---|---|
| CAS | 105-57-7 |
| Melting Point | -100 |
| Density | 0.8254 |
| Molecular Weight | mol. wt. = 118.18 |
| Refractive Index | 1.379-1.385 |
| Boiling Point | 102.7 |
Hazards and Safety
Highly flammable. Flash point = -12C. Sensitive to light, moisture and heat. May form explosive peroxides. Contact causes irritation. Narcotic in high concentrations.
Fisher Scientific: MSDS
Authority
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Website address 1 Comment: Fisher Scientific at https://www1.fishersci.com/catalogs/acrosgroup.jsp?catalogParamId=8013176&catalogParamType=AG Flash point = -21C