Difference between revisions of "Aluminon"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 42: | Line 42: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 330 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 330 | ||
| Line 48: | Line 48: | ||
* ''A Glossary of Paper Conservation Terms'', Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998 | * ''A Glossary of Paper Conservation Terms'', Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998 | ||
| − | * | + | * N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'', Archetype Publications, London, 2000 |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution= 1 g salt in 1 liter distilled water | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution= 1 g salt in 1 liter distilled water | ||
Revision as of 06:25, 24 July 2013
Description
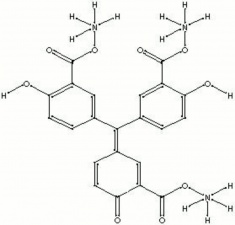
A tradename for shiny, yellow-brown powder that is the ammonium salt of aurin tricarboxylic acid. Aluminon forms brightly colored lakes with aluminum, chromium, iron, and beryllium salts. It is generally used for the detection of alum in aqueous extractions of paper.
Synonyms and Related Terms
ammonium salt of aurine tricarboxylic acid; Lysofon; ammonium aurintricarboxylate;
Other Properties
Soluble in water.
The reagent solution is prepared by dissolving 1 g of salt in 1 liter of distilled water.
| Composition | C22H23N3O9 |
|---|---|
| CAS | 569-58-4 |
| Melting Point | 220.5 (dec) |
| Molecular Weight | mol. wt. = 473.43 |
Hazards and Safety
Skin contact causes irritation. Harmful if swallowed or inhaled.
Mallinckrodt Baker: MSDS
Additional Information
For alum detection procedures see: N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology Archetype Publications, London, 2000, p. 34.
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 330
- A Glossary of Paper Conservation Terms, Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998
- N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology, Archetype Publications, London, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: Solution= 1 g salt in 1 liter distilled water