Difference between revisions of "Tungsten trioxide"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 39: | Line 39: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 694 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9946 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9946 | ||
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
| − | * | + | * Robert Fournier, ''Illustrated Dictionary of Practical Pottery'', Chilton Book Company, Radnor, PA, 1992 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:25, 24 July 2013
Description
Yellow powder used as for fireproofing textiles. Tungsten trioxide, or anhydrous tungstic acid, is also used as a high temperature yellow pigment in ceramics.
Synonyms and Related Terms
anhydrous tungstic acid; anhydrous wolframic acid; tungstic oxide; tungsten oxide
Other Properties
Soluble in hydrofluoric acid and alkalis.
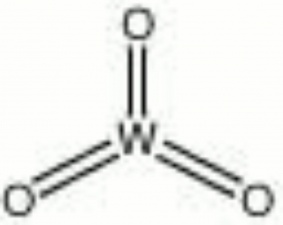
| Composition | WO3 |
|---|---|
| CAS | 1314-35-8 |
| Melting Point | 1473 |
| Density | 7.16 |
| Molecular Weight | mol. wt. = 231.86 |
Hazards and Safety
Toxic by ingestion and inhalation. Noncombustible.
Fisher Scientific: MSDS
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 694
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9946
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Robert Fournier, Illustrated Dictionary of Practical Pottery, Chilton Book Company, Radnor, PA, 1992