Difference between revisions of "Morpholine"
(username removed) |
(username removed) |
||
| Line 44: | Line 44: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6362 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6362 | ||
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
| − | * | + | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 |
| − | * | + | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 |
| − | * | + | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:28, 24 July 2013
Description
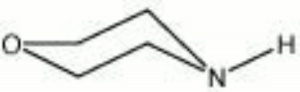
A colorless, hygroscopic liquid with a characteristic amine odor. Morpholine is miscible with water, but it evolves heat in the process forming a basic solution that can saponify dried oil films and aid in their removal. Morpholine is also used to dissolve resins, waxes, casein, shellac, and dyes. It can also act as a surfactant and emulsifier. Morpholine has been used as a corrosion inhibitor in fire sprinkler and HVAC systems. It has also been used as a vapor phase neutralizing/alkalizing agent, but it is not recommended because of its toxicity, disagreeable odor, and poor ability to provide residual alkalinity. Some materials treated with morpholine, such as leather and pyroxylin coated book covers, have exhibited color changes (Book and Paper Catalog).
Synonyms and Related Terms
tetrahydro-1,4-oxazine; diethylene oximide; diethylene imidoxide
Other Properties
Soluble in water and organic solvents.
Vapor pressure = 8.0 at 20C
| Composition | C4H8ONH |
|---|---|
| CAS | 110-91-8 |
| Melting Point | -4.9 |
| Density | 1.007 |
| Molecular Weight | mol. wt. = 87.1 |
| Boiling Point | 128.9 |
Hazards and Safety
Flammable, moderate fire risk. Flash point = 38C Toxic by inhalation, ingestion and skin absorption. Skin contact causes irritation.
LINK: International Chemical Safety Card
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6362
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989