Difference between revisions of "Sulfamic acid"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White, crystalline solid. Sulfamic acid is used as a flame retardant for textiles and wood, buffer, acid cleaner, chlorine stabilizer in swimming pools, nitrite scavenger, and sulfonating agent. It is also used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=bleaching | + | White, crystalline solid. Sulfamic acid is used as a flame retardant for textiles and wood, buffer, acid cleaner, chlorine stabilizer in swimming pools, nitrite scavenger, and sulfonating agent. It is also used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=bleaching%20agent bleaching agent] for paper pulp, textiles, and color photographs. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 41: | Line 41: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 782 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
* ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | * ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | ||
Revision as of 06:30, 24 July 2013
Description
White, crystalline solid. Sulfamic acid is used as a flame retardant for textiles and wood, buffer, acid cleaner, chlorine stabilizer in swimming pools, nitrite scavenger, and sulfonating agent. It is also used as a bleaching agent for paper pulp, textiles, and color photographs.
Synonyms and Related Terms
amidosulfonic acid; amidosulfuric acid; sulfamidic acid
Other Properties
Soluble in water, but hydrolyzed in water to form ammonium bisulfate. Slightly soluble in organic solvents.
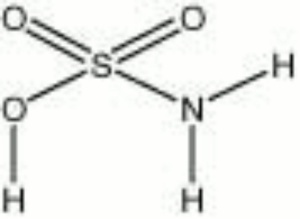
| Composition | SOOHNH2 |
|---|---|
| CAS | 5329-14-6 |
| Melting Point | 205 (dec) |
| Density | 2.15 |
| Molecular Weight | mol. wt. = 97.1 |
Hazards and Safety
Toxic by ingestion. Corrosive to eyes, skin and lungs causing irritation and burns.
Heating results in the production of toxic sulfur dioxide fumes.
LINK: International Chemical Safety Card
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 782
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9090