Difference between revisions of "Lithium carbonate"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white crystalline compound. Lithium carbonate is used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=flux flux] in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=ceramic ceramic] and [http://cameo.mfa.org/materials/fullrecord.asp?name=porcelain porcelain] [http://cameo.mfa.org/materials/fullrecord.asp?name=glaze glazes], and [http://cameo.mfa.org/materials/fullrecord.asp?name=enamel | + | A white crystalline compound. Lithium carbonate is used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=flux flux] in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=ceramic ceramic] and [http://cameo.mfa.org/materials/fullrecord.asp?name=porcelain porcelain] [http://cameo.mfa.org/materials/fullrecord.asp?name=glaze glazes], and [http://cameo.mfa.org/materials/fullrecord.asp?name=enamel%2C%20inorganic enamels]. It is also used to make [http://cameo.mfa.org/materials/fullrecord.asp?name=luminescence luminescent] [http://cameo.mfa.org/materials/fullrecord.asp?name=paint paints], [http://cameo.mfa.org/materials/fullrecord.asp?name=varnish varnishes], and [http://cameo.mfa.org/materials/fullrecord.asp?name=dye dyes]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 42: | Line 42: | ||
== Authority == | == Authority == | ||
| − | * | + | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5552 |
| − | * | + | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
| − | * | + | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:31, 24 July 2013
Description
A white crystalline compound. Lithium carbonate is used as a flux in the manufacture of ceramic and porcelain glazes, and enamels. It is also used to make luminescent paints, varnishes, and dyes.
Synonyms and Related Terms
dilithium carbonate; Camcolit; Candamide; Carbolith; Eskalith; Limas; Lithane; Lithobid; Lithonate; Lithotabs; Plenur; Priadel
Other Properties
Soluble in dilute acid. Slightly soluble in water. Insoluble in ethanol.
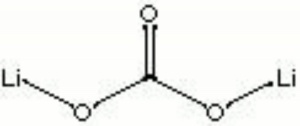
| Composition | Li2CO3 |
|---|---|
| CAS | 554-13-2 |
| Melting Point | 720 |
| Density | 2.111 |
| Molecular Weight | mol. wt. = 73.9 |
| Boiling Point | 1200(dec) |
Hazards and Safety
Corrosive to skin, eyes, and membranes.
LINK: International Chemical Safety Card
Authority
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 5552
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979