Difference between revisions of "Nitric acid"
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A colorless, viscous, strongly oxidizing liquid that turns yellow with age or light exposure due to the formation of nitric oxides. Nitric acid is sold in concentrations ranging from >90% (fuming) down to 36%. The commercial reagent grade of nitric acid used in most labs contains 70% nitric acid in water. Nitric acid will dissolve most [http://cameo.mfa.org/materials/fullrecord.asp?name=metal metals]. It is used in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=ammonium | + | A colorless, viscous, strongly oxidizing liquid that turns yellow with age or light exposure due to the formation of nitric oxides. Nitric acid is sold in concentrations ranging from >90% (fuming) down to 36%. The commercial reagent grade of nitric acid used in most labs contains 70% nitric acid in water. Nitric acid will dissolve most [http://cameo.mfa.org/materials/fullrecord.asp?name=metal metals]. It is used in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=ammonium%20nitrate ammonium nitrate] for fertilizer and explosives. It is also used for metallurgy, photoengraving, etching [http://cameo.mfa.org/materials/fullrecord.asp?name=steel steel], and the synthesis of [http://cameo.mfa.org/materials/fullrecord.asp?name=dye dyes] and [http://cameo.mfa.org/materials/fullrecord.asp?name=polymer polymers]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | aqua fortis; engraver's acid; azotic acid; spirits of nitre; azotic acid; hydrogen nitrate; salpetersyre (Dan., Nor.); | + | aqua fortis; engraver's acid; azotic acid; spirits of nitre; azotic acid; hydrogen nitrate; salpetersyre (Dan., Nor.); Salpetersäure (Deut.); ácido nítrico (Esp., Port.); ácido trioxonítrico (V) (Esp.); acide nitrique (Fr.); acido nitrico (It.); salpeterzuur (Ned.); Kwas azotowy(V) (Pol.); salpetersyra (Sven.) |
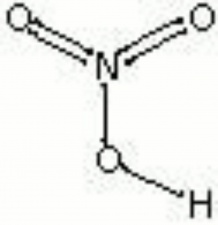
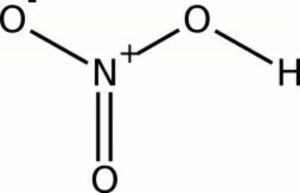
[[[SliderGallery rightalign|nitric acid.jpg~Chemical structure|Nitricacidvt.jpg~Chemical structure]]] | [[[SliderGallery rightalign|nitric acid.jpg~Chemical structure|Nitricacidvt.jpg~Chemical structure]]] | ||
| Line 44: | Line 44: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 545 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 |
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6672 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6672 | ||
| Line 60: | Line 60: | ||
* Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Nitric_acid (Accessed Jan. 15, 2006) | * Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Nitric_acid (Accessed Jan. 15, 2006) | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Revision as of 06:32, 24 July 2013
Description
A colorless, viscous, strongly oxidizing liquid that turns yellow with age or light exposure due to the formation of nitric oxides. Nitric acid is sold in concentrations ranging from >90% (fuming) down to 36%. The commercial reagent grade of nitric acid used in most labs contains 70% nitric acid in water. Nitric acid will dissolve most metals. It is used in the manufacture of ammonium nitrate for fertilizer and explosives. It is also used for metallurgy, photoengraving, etching steel, and the synthesis of dyes and polymers.
Synonyms and Related Terms
aqua fortis; engraver's acid; azotic acid; spirits of nitre; azotic acid; hydrogen nitrate; salpetersyre (Dan., Nor.); Salpetersäure (Deut.); ácido nítrico (Esp., Port.); ácido trioxonítrico (V) (Esp.); acide nitrique (Fr.); acido nitrico (It.); salpeterzuur (Ned.); Kwas azotowy(V) (Pol.); salpetersyra (Sven.)
Other Properties
Soluble in water. Decomposes in ethanol. Dissolves most metals.
| Composition | HNO3 |
|---|---|
| CAS | 7697-37-2 |
| Melting Point | -41.59 |
| Density | 1.504 (95%) |
| Molecular Weight | mol. wt. = 63.0 |
| Boiling Point | 78 (dec) |
Hazards and Safety
Strong oxidizing agent. Dangerous fire risk in contact with organic materials.
Highly toxic by inhalation, corrosive to skin.
LINK: International Chemical Safety Card
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 545
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6672
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Nitric_acid (Accessed Jan. 15, 2006)
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976