Difference between revisions of "Tetrachloroethylene"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 47: | Line 47: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 |
| − | * | + | * Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9332; ref. index=1.5055 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9332; ref. index=1.5055 | ||
Revision as of 06:34, 24 July 2013
Description
Colorless, chlorinated hydrocarbon with an ether-like odor. Tetrachloroethylene (TCE) was first prepared by Faraday in 1921. It is currently used as a dry-cleaning solvent and as a vapor-degreaser for metals. TCE is a suspected carcinogen.
Synonyms and Related Terms
TCE; tetrachloroethene; ethylene tetrachloride; perchloroethylene; Perclene; Vaclene [DuPont]
Other Properties
Miscible in ethanol, ether, chloroform, benzene. Insoluble in water.
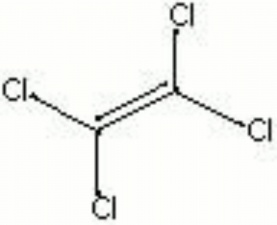
| Composition | Cl2C:CCl2 |
|---|---|
| CAS | 127-18-4 |
| Melting Point | 22 |
| Density | 1.6230 |
| Molecular Weight | mol. wt.= 165.83 . |
| Refractive Index | 1.5055 |
| Boiling Point | 121 |
Hazards and Safety
Irritating to eyes and skin. Potential carcinogen. Dangerous to the environment.
Nonflammable, but may decompose in the presence of flames or UV light to form toxic fumes (phosgene, hydrogen chloride).
LINK: International Chemical Safety Card
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9332; ref. index=1.5055
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.504