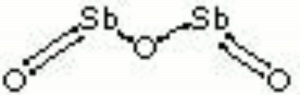Difference between revisions of "Antimony trioxide"
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white crystalline powder that occurs in nature as the mineral valentinite. Synthetic antimony trioxide, produced by roasting [http://cameo.mfa.org/materials/fullrecord.asp?name=antimony antimony] ore then mixing with [http://cameo.mfa.org/materials/fullrecord.asp?name=barium | + | A white crystalline powder that occurs in nature as the mineral valentinite. Synthetic antimony trioxide, produced by roasting [http://cameo.mfa.org/materials/fullrecord.asp?name=antimony antimony] ore then mixing with [http://cameo.mfa.org/materials/fullrecord.asp?name=barium%20sulfate barium sulfate] was introduced as an artists' pigment called antimony white and Timonox in 1919. It is inert, has good hiding power, and low oil absorption. Since it is darkened by [http://cameo.mfa.org/materials/fullrecord.asp?name=hydrogen%20sulfide hydrogen sulfide], antimony oxide is often mixed with [http://cameo.mfa.org/materials/fullrecord.asp?name=zinc%20oxide zinc oxide] which has preferential absorption for that gas (Gettens and Stout 1966). Some samples may contain senarmonite and/or valentinite, two known mineral forms of antimony oxide. Octahedral arsenic oxide may also be present as an impurity. Antimony trioxide is used as a white pigment and opacifiers in waxes, enamels, and glasses. It is also used to flameproof textiles, paper, and plastic. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | antimony (III) oxide; antimony white; antimony oxide; Pigment White 11; Antimontrioxid (Deut.); Antimonweiss (Deut.); blanc d'antimoine (Fr.); oxyde d'antimoine (Fr.); leyko toy antimonioy (Gr.); bianco di antimonio (It.); triossido d'antimonio (It.); antimoon wit (Ned.); | + | antimony (III) oxide; antimony white; antimony oxide; Pigment White 11; Antimontrioxid (Deut.); Antimonweiss (Deut.); blanc d'antimoine (Fr.); oxyde d'antimoine (Fr.); leyko toy antimonioy (Gr.); bianco di antimonio (It.); triossido d'antimonio (It.); antimoon wit (Ned.); trióxido de antimónio (Port.); diantimony trioxide; antimony sesquioxide; flowers of antimony; exitelite; senarmontite; valentinite; weisspiessglanz; Timonox [Cookson Lead and Antimony, England]; |
[[[SliderGallery rightalign|antimony trioxide.jpg~Chemical structure]]] | [[[SliderGallery rightalign|antimony trioxide.jpg~Chemical structure]]] | ||
| Line 47: | Line 47: | ||
== Additional Information == | == Additional Information == | ||
| − | R. J. Gettens and G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966. | + | ° R. J. Gettens and G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966. |
== Comparisons == | == Comparisons == | ||
| Line 57: | Line 57: | ||
== Authority == | == Authority == | ||
| − | * | + | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: refractive index=2.087. Date for Timonox=1919 |
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 5.75 and ref. index = 2.18;2.35 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 64 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 752 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 752 | ||
Revision as of 06:48, 24 July 2013
Description
A white crystalline powder that occurs in nature as the mineral valentinite. Synthetic antimony trioxide, produced by roasting antimony ore then mixing with barium sulfate was introduced as an artists' pigment called antimony white and Timonox in 1919. It is inert, has good hiding power, and low oil absorption. Since it is darkened by hydrogen sulfide, antimony oxide is often mixed with zinc oxide which has preferential absorption for that gas (Gettens and Stout 1966). Some samples may contain senarmonite and/or valentinite, two known mineral forms of antimony oxide. Octahedral arsenic oxide may also be present as an impurity. Antimony trioxide is used as a white pigment and opacifiers in waxes, enamels, and glasses. It is also used to flameproof textiles, paper, and plastic.
Synonyms and Related Terms
antimony (III) oxide; antimony white; antimony oxide; Pigment White 11; Antimontrioxid (Deut.); Antimonweiss (Deut.); blanc d'antimoine (Fr.); oxyde d'antimoine (Fr.); leyko toy antimonioy (Gr.); bianco di antimonio (It.); triossido d'antimonio (It.); antimoon wit (Ned.); trióxido de antimónio (Port.); diantimony trioxide; antimony sesquioxide; flowers of antimony; exitelite; senarmontite; valentinite; weisspiessglanz; Timonox [Cookson Lead and Antimony, England];
Other Properties
Soluble in concentrated hydrochloric and sulfuric acids, strong alkalis. Insoluble in water.
Fine crystals (about 1 micron) appearing rounded or cubic. Colorless in plane-polarized light. Isotropic with low birefringence.
| Composition | Sb2O3 |
|---|---|
| CAS | 1309-64-4 |
| Melting Point | 655 |
| Density | 5.67-5.75 |
| Molecular Weight | mol. wt. = 291.5 |
| Refractive Index | 2.18; 2.35 |
| Boiling Point | 1425 |
Hazards and Safety
Highly toxic by inhalation and ingestion. Skin contact is corrosive. Fumes are carcinogenic.
International Chemical Safety Card
Additional Information
° R. J. Gettens and G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966.
Comparisons
Characteristics of Common White Pigments
Authority
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: refractive index=2.087. Date for Timonox=1919
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 5.75 and ref. index = 2.18;2.35
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 64
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 752
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000