Difference between revisions of "Chromium oxide hydrate"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 6: | Line 6: | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | viridian; transparent chrome oxide; Pigment Green 18; CI 77289; | + | viridian; transparent chrome oxide; Pigment Green 18; CI 77289; hidróxido de cromo (Esp.); hydroxyde de chrome (Fr.); idrossido di cromo (It.); hidróxido de crómio (Port.); chromic hydrate; chromic hydroxide; chromium hydroxide; chromium hydrate; Guignet's green; chromic oxide gel; hydrous chromic oxide |
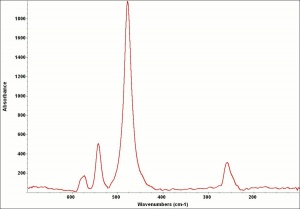
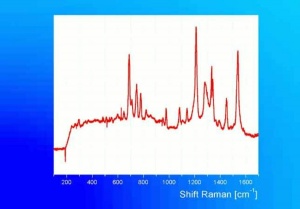
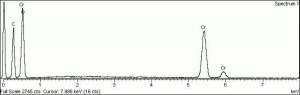
[[[SliderGallery rightalign|ViridianUCL.jpg~Raman|viridian632.jpg~Raman|f412sem.jpg~SEM|f412edsbw.jpg~EDS]]] | [[[SliderGallery rightalign|ViridianUCL.jpg~Raman|viridian632.jpg~Raman|f412sem.jpg~SEM|f412edsbw.jpg~EDS]]] | ||
| Line 29: | Line 29: | ||
== Authority == | == Authority == | ||
| − | * | + | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2281 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2281 | ||
Revision as of 06:49, 24 July 2013
Description
A transparent, green gelatinous mass. Chromium oxide hydrate, commonly called viridian, is used as a pigment, a tanning agent and as a mordant for textile dyeing.
Synonyms and Related Terms
viridian; transparent chrome oxide; Pigment Green 18; CI 77289; hidróxido de cromo (Esp.); hydroxyde de chrome (Fr.); idrossido di cromo (It.); hidróxido de crómio (Port.); chromic hydrate; chromic hydroxide; chromium hydroxide; chromium hydrate; Guignet's green; chromic oxide gel; hydrous chromic oxide
Other Properties
Soluble in acid and alkali. Insoluble in water. Decomposes to chromic oxide with heat.
| Composition | Cr(OH)3 |
|---|---|
| Molecular Weight | mol. wt. = 103.02 |
Hazards and Safety
Toxic by ingestion, inhalation and skin contact. Suspected carcinogen..
Authority
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 2281