Difference between revisions of "Scheele's green"
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A highly poisonous green pigment composed of [http://cameo.mfa.org/materials/fullrecord.asp?name=copper | + | A highly poisonous green pigment composed of [http://cameo.mfa.org/materials/fullrecord.asp?name=copper%20arsenite copper arsenite]. Scheele's green was discovered in Sweden in 1775 by Carl W. Scheele, a German chemist, but he did not publish the recipe until 1778. It is a bright, warm yellowish-green with good opacity. Scheele's green was never widely used as a paint pigment because it was toxic and discolored in the presence of acid or sulfur fumes. Currently, copper arsenite is used as a rodenticide, [http://cameo.mfa.org/materials/fullrecord.asp?name=insecticide insecticide], fungicide, and wood preservative. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | copper arsenite; cupric arsenite; Pigment Green 22; CI 77412; mineral green; ash green; vert de Scheele (Fr.); | + | copper arsenite; cupric arsenite; Pigment Green 22; CI 77412; mineral green; ash green; vert de Scheele (Fr.); Scheelesgrün (Deut.); verde di Scheele (It.) |
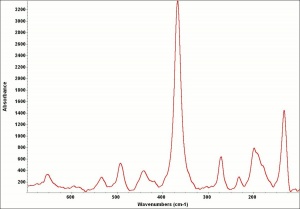
[[[SliderGallery rightalign|ScheelesUCL.jpg~Raman]]] | [[[SliderGallery rightalign|ScheelesUCL.jpg~Raman]]] | ||
| Line 38: | Line 38: | ||
== Authority == | == Authority == | ||
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * R.D. Harley, ''Artists' Pigments c. 1600-1835'', Butterworth Scientific, London, 1982 |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * | + | * R. Newman, E. Farrell, 'House Paint Pigments', ''Paint in America '', R. Moss ed., Preservation Press, New York City, 1994 |
* ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigments' | * ''The Dictionary of Art'', Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigments' | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 610 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 |
| − | * | + | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 06:50, 24 July 2013
Description
A highly poisonous green pigment composed of copper arsenite. Scheele's green was discovered in Sweden in 1775 by Carl W. Scheele, a German chemist, but he did not publish the recipe until 1778. It is a bright, warm yellowish-green with good opacity. Scheele's green was never widely used as a paint pigment because it was toxic and discolored in the presence of acid or sulfur fumes. Currently, copper arsenite is used as a rodenticide, insecticide, fungicide, and wood preservative.
Synonyms and Related Terms
copper arsenite; cupric arsenite; Pigment Green 22; CI 77412; mineral green; ash green; vert de Scheele (Fr.); Scheelesgrün (Deut.); verde di Scheele (It.)
Other Properties
Soluble in mineral acids. Insoluble in water.
Decomposes in alkalis. Darkens in the presence of sulfur or lead compounds.
| Composition | Cu(AsO2)2 |
|---|---|
| Refractive Index | 1.55 - 1.75 |
Hazards and Safety
Extremely toxic by ingestion, inhalation and skin absorption.
Human carcinogen.
May produce toxic arsenic fumes when decomposed by fungi.
Additional Information
I.Fiedler, M Bayard, "Emerald Green and Scheele's Green", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.
Authority
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- R. Newman, E. Farrell, 'House Paint Pigments', Paint in America , R. Moss ed., Preservation Press, New York City, 1994
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigments'
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 610
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989