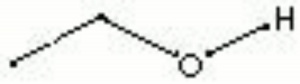Difference between revisions of "Ethyl alcohol"
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 51: | Line 51: | ||
| − | == | + | == Sources Checked for Data in Record == |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Revision as of 20:20, 30 April 2016
Description
A clear, colorless, hygroscopic liquid with a pleasant odor. Ethyl alcohol, or ethanol, is primarily used as a solvent and as an intoxicating beverage. Ethanol forms a binary azeotrope with water that boils at 78.15C and contains 95.57% ethanol and 4.43% water. The addition of benzene allows the mixture to be redistilled without the water. Ethyl alcohol is sold in many grades marked as 95%, absolute (100% or anhydrous), denatured, industrial, or listed as proofs (one-half the proof is the percentage of alcohol). In art and conservation, ethanol has been used as a solvent for shellac and mastic, as a diluent for fixatives, and as a wetting agent. When used as a solvent for resins, the ethanol must be dry because any moisture will produce a white haze in the varnish film.
Synonyms and Related Terms
ethanol (IUPAC); alcohol; grain alcohol; absolute alcohol, EtOH, anhydrous alcohol; dehydrated alcohol; ethyl hydrate; ethyl hydroxide; Cologne spirits; colonial spirits; rectified spirits; spirits of wine; fermentation alcohol
Other Properties
Miscible with water, methanol, ether, chloroform, acetone.
| Composition | C2H5OH |
|---|---|
| CAS | 64-17-5 |
| Melting Point | -114.1 |
| Density | 0.789 |
| Molecular Weight | mol. wt.= 46.08 |
| Refractive Index | 1.359 |
| Boiling Point | 78.5 |
Hazards and Safety
Highly flammable. Flash point = 14 C (60F).
Inhalation, and skin contact can cause irritation. Ingestion of small amounts affects the central nervous system. Ingestion of large amounts is deadly.
International Chemical Safety Card
Comparisons
Sources Checked for Data in Record
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 3806
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.359