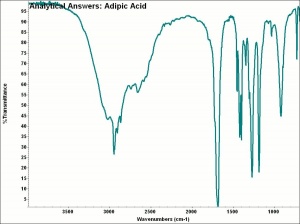
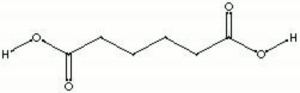
Difference between revisions of "Adipic acid"
(username removed) |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white crystalline, solid that occurs naturally in beet juice. Adipic acid is prepared synthetically from cyclohexanol. It is primarily used in the production of [ | + | A white crystalline, solid that occurs naturally in beet juice. Adipic acid is prepared synthetically from cyclohexanol. It is primarily used in the production of [[nylon%20resin|nylon]] and [[polyurethane|polyurethane]] foams. Adipic acid is also used as a [[plasticizer|plasticizer]], [[lubricant|lubricant]], and a food additive in baking powder (in place of [[cream%20of%20tartar|cream of tartar]]) and in beverages (in place of [[citric%20acid|citric acid]]). It is not [[hygroscopic|hygroscopic]]. Prior to 1940, adipic acid was also used for bronzing metals, preparing photographic paper, textile dyeing, and as a component in synthetic wax sizes mixed with [[glycerol|glycerol]], [[stearic%20acid|stearic acid]], and [[palmitic%20acid|palmitic acid]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 14:31, 6 January 2014
Description
A white crystalline, solid that occurs naturally in beet juice. Adipic acid is prepared synthetically from cyclohexanol. It is primarily used in the production of nylon and Polyurethane foams. Adipic acid is also used as a Plasticizer, Lubricant, and a food additive in baking powder (in place of Cream of tartar) and in beverages (in place of Citric acid). It is not Hygroscopic. Prior to 1940, adipic acid was also used for bronzing metals, preparing photographic paper, textile dyeing, and as a component in synthetic wax sizes mixed with Glycerol, Stearic acid, and Palmitic acid.
Synonyms and Related Terms
hexanedioic acid; 1,4-butanedicarboxylic acid; adipinic acid' Adipinsäure (Deut.); acide adipique (Fr.); acide 1,6-hexanedioïque (Fr.);
Other Properties
Soluble in methanol, ethanol, ethyl acetate, acetone. Slightly soluble in water, cyclohexane. Insoluble in benzene, ligroin.
pH of a saturated solution is 2.7
| Composition | COOH(CH2)4COOH |
|---|---|
| CAS | 124-04-9 |
| Melting Point | 152 |
| Density | 1.360 |
| Molecular Weight | mol. wt. = 146.14 |
| Boiling Point | 337.5 |
Hazards and Safety
International Chemical Safety Card
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 17
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 161