Difference between revisions of "Butyric acid"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 45: | Line 45: | ||
Fisher Scientific: [https://fscimage.fishersci.com/msds/03680.htm MSDS] | Fisher Scientific: [https://fscimage.fishersci.com/msds/03680.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
* Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | * Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | ||
Revision as of 13:18, 29 April 2016
Description
A colorless liquid with a rancid butter smell. Butyric acid occurs naturally in butter in concentration of about 5%. It is used in the manufacture of varnishes and the pretreatment of hides for tanning.
Synonyms and Related Terms
butanoic acid; n-butyric acid; ethylacetic acid; propylformic acid
Other Properties
Miscible with water, ethanol, ether.
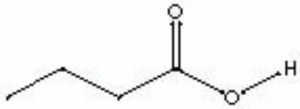
| Composition | CH3CH2CH2COOH |
|---|---|
| CAS | 107-92-6 |
| Melting Point | -7.9 |
| Density | 0.959 |
| Molecular Weight | mol. wt. = 88.11 |
| Refractive Index | 1.3981 |
| Boiling Point | 163.5 |
Hazards and Safety
Combustible. Flash point = 69C.
Strongly irritating to skin and tissue. May cause burns.
Fisher Scientific: MSDS
Sources Checked for Data in Record
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 343
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1566; ref. index=1.3981
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.396