Difference between revisions of "Nitric acid"
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| Line 42: | Line 42: | ||
LINK: [http://www.cdc.gov/niosh/ipcsneng/neng0183.html International Chemical Safety Card] | LINK: [http://www.cdc.gov/niosh/ipcsneng/neng0183.html International Chemical Safety Card] | ||
| − | == | + | == Sources Checked for Data in Record == |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 545 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 545 | ||
Revision as of 13:11, 1 May 2016
Description
A colorless, viscous, strongly oxidizing liquid that turns yellow with age or light exposure due to the formation of nitric oxides. Nitric acid is sold in concentrations ranging from >90% (fuming) down to 36%. The commercial reagent grade of nitric acid used in most labs contains 70% nitric acid in water. Nitric acid will dissolve most metals. It is used in the manufacture of ammonium nitrate for fertilizer and explosives. It is also used for metallurgy, photoengraving, etching steel, and the synthesis of dyes and polymers.
Synonyms and Related Terms
aqua fortis; engraver's acid; azotic acid; spirits of nitre; azotic acid; hydrogen nitrate; salpetersyre (Dan., Nor.); Salpetersäure (Deut.); ácido nítrico (Esp., Port.); ácido trioxonítrico (V) (Esp.); acide nitrique (Fr.); acido nitrico (It.); salpeterzuur (Ned.); Kwas azotowy(V) (Pol.); salpetersyra (Sven.)
Other Properties
Soluble in water. Decomposes in ethanol. Dissolves most metals.
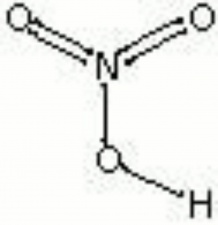
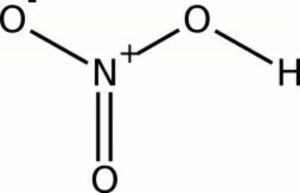
| Composition | HNO3 |
|---|---|
| CAS | 7697-37-2 |
| Melting Point | -41.59 |
| Density | 1.504 (95%) |
| Molecular Weight | mol. wt. = 63.0 |
| Boiling Point | 78 (dec) |
Hazards and Safety
Strong oxidizing agent. Dangerous fire risk in contact with organic materials.
Highly toxic by inhalation, corrosive to skin.
LINK: International Chemical Safety Card
Sources Checked for Data in Record
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 545
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6672
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Nitric_acid (Accessed Jan. 15, 2006)
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976